Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 11/11/2006
7. Ligand-Androgen Receptor Complexes
Aede de Groot, Willem Koert
In chapter 4 we have seen how carbon atoms are assembled to molecules of the steroid hormone testosterone and the shape of this molecule has been discussed in detail. The spatial structural formula shows that testosterone has a relatively flat skeleton of three six membered rings and one five membered ring. Several substituents are attached to this skeleton. At C3 there is a carbonyl group, at C17 a b-hydroxyl group, and at C10 and C13 there are methyl groups (C19 and C18). Between C4 and C5 there is a double bond so that there is a flat region in the molecule around these atoms (see Figure 1).
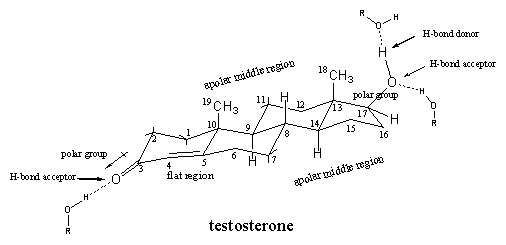
Figure 1
Next to the shape of the molecule, also polar and apolar regions can be distinguished. The carbonyl group at C3 is a polar group in which the O-atom is slightly negatively charged and the C3-atom is a bit positive. Also the hydroxyl group at C17 has a polar character with the O-atom as the negative centre.
Both O-containing groups also have the capability to participate in hydrogen bonding. The carbonyl group can act only as a hydrogen bond acceptor, the hydroxyl group is a hydrogen bond acceptor with the O-atom, and a hydrogen bond donor, with the H-atom that is attached to this O-atom.
The top side of the steroid skeleton with the two methyl groups and the middle part are the apolar regions of the molecule, which can interact through Van der Waals forces (apolar interactions) with apolar side chains of the amino acids inside the middle of the receptor pocket.
The steroid hormones that interact with the receptor are called ligands, so testosterone is the natural ligand for the androgen receptor. Mutual interactions on a molecular level determine the bonding between ligand and receptor. The androgen receptor is a large protein molecule with a pocket inside in which the ligand fits. This pocket has a shape, which is complementary to the shape of the ligand molecule. Next to that, there are polar and apolar interactions and hydrogen bonding at work between the side chains of the amino acids inside the pocket and the ligand, to achieve bonding. In chapter 6 the underlying principles of polar and apolar interactions and hydrogen bonding between molecules have been discussed.
In chapter 3 we have shown that a protein is constructed from amino acids that form a long chain. In the androgen receptor 919 amino acids are coupled together, but slight variations in this number may occur in different individuals. These 919 amino acids together make a giant molecule with a molecular weight of about 110000 D units.
Through interactions between the peptide bonds and between the side chains of the amino acids, this chain is folded in a characteristic way to a kind of loose ball. In this ball different areas, called domains, are distinguished. Each domain has its own place and function.
The molecular weight of the ligand testosterone is 288 D units and it is clear from these molecular weights that the ligand testosterone is much smaller than the androgen receptor.
It is indeed so that only part of the receptor is necessary to form the pocket in which testosterone has to fit. This part of the receptor is called the ligand bonding domain (LBD). In the androgen receptor this domain is constructed from approximately 256 amino acids which are located on the places 663-919 in the protein chain.
Other domains of the receptor are for bonding of the receptor-ligand complex to the DNA. This interaction is necessary to initiate protein synthesis. In chapter 8 more will be told about the function of the different domains of the androgen receptor. In this chapter we will concentrate on the function of the ligand binding domain and to bonding of the ligand.
It is reasonably clear which factors play a role in bonding between the ligand and the androgen receptor. This is important because the formation of the ligand-receptor complex is an essential step in the whole process of muscle protein synthesis. As soon as bonding between the ligand and the receptor has taken place, the shape of the receptor-ligand complex changes dramatically. This enables the complex to free itself from accompanying proteins to allow transport to the cell nucleus where it has to interact with DNA.
The side chains of the amino acids that line the inside of the pocket are essential for bonding of the ligand. The strength of this bonding is much weaker then that of a normal covalent bonding between two atoms in a molecule. The strength of a normal C-C bond is 88 kcal/mole, while the strength of the interaction between a ligand and its receptor is estimated to be 30-40 kcal/mole. This weaker interaction is necessary because it is not the intention that the bonding of a ligand to its receptor is permanent. This bonding should be established and broken when necessary. On the other hand the interaction should also not be so weak that hardly any bonding takes place.
In the pocket of the androgen receptor 18 amino acids play a role in bonding of the ligand. These amino acids are mostly not situated next to each other in the protein chain but after folding of this chain they come together in the inside of the pocket.
For four combinations of the ligand bonding domain of the androgen receptor and ligand it is now known how bonding takes place. This can be investigated with X-ray crystallography of the ligand bonding domain-ligand complex. This is not a simple task and a difficult step in the whole process is to obtain good crystals of the ligand-receptor complex. Large proteins usually do not crystallize easily. Also for this reason there is still is no X-ray known of the whole androgen receptor-ligand complex.
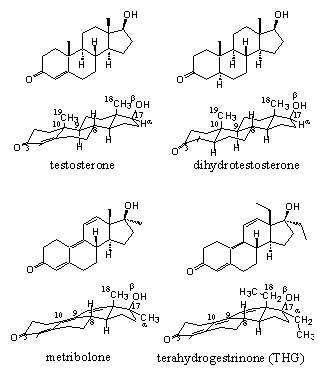 Figure 2 |
In 2006 an X-ray has been determined for the complex of testosterone with the ligand bonding domain of the androgen receptor. [1] There is also an X-ray determined of the complex of the other natural ligand dihydrotestosterone [2] with the ligand binding domain of the androgen receptor. X-rays of the complexes of the anabolic steroid metribolone [3] [4] and of the designer steroid tetrahydrogestrinone (THG) [1] with the ligand binding domain of the androgen receptor have been determined also. The latter two steroids have a slightly different interaction with the receptor than testosterone and dihydrotestosterone.
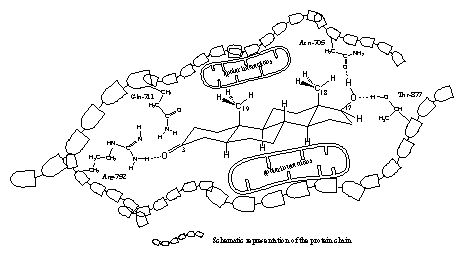
It appears from the X-rays that in all cases a hydrogen bond is present between the carbonyl group at C3 and the side chain of the amino acid arginine, located at place 752 (Arg-752) in the protein chain. The amino group of Arg-752 is the hydrogen bond donor and the O-atom of the carbonyl group is the hydrogen bond acceptor (see Figure 3).
The X-ray’s of the testosterone- and dihydrotestosterone-ligand binding domain complexes also indicate a role for the amino acid glutamine at place 711 in the protein chain (Glu-711) in the bonding of the ligand. In the THG- and metribolone-ligand bonding domain complexes this is not the case. The flatter structures of THG and metribolone should be responsible for this difference. In all cases one molecule of water, which is present in the pocket in the neighbourhood of the carbonyl group, plays a role in the bonding (this is not indicated in figure 3).
The flatter structures of THG and metribolone also slightly change the angle of the hydrogen bond between the carbonyl group in these molecules and the side chain of arginine. The strength of a hydrogen bond is dependant on this angle. The strongest hydrogen bond bonds are linear, so with an angle of 180° between the bonds.
Figure 3
Click for larger pic
In all X-rays hydrogen bonding is found between the H-atom of the hydroxyl group at C17 as donor and the O-atom of the amide group in the side chain of asparagine at place 705 in the protein chain (Asp-705) as hydrogen bond acceptor.
This C17 hydroxyl group forms a second hydrogen bond, now with its O-atom as acceptor and with the hydroxyl group of the amino acid threonine at place 877 (Thr-877) in the protein chain as hydrogen bond donor.
The apolar side chains of the amino acids in the middle of the pocket have apolar Van der Waals interactions with the apolar middle part of the steroid skeleton.
We have tried to represent these interactions for dihydrotestosterone and the ligand binding domain of the receptor in a schematic way in a flat drawing in Figure 3. This is not so easy because in reality the pocket and dihydrotestosterone are three dimensional. It does however help to imagine which factors play a role in the interactions between a steroid ligand and the androgen receptor.
Important are the shape of the ligand and the pocket, the carbonyl group at C3 and the hydroxyl group at C17, factors that have been recognized already for a long time.
Recent X-rays of the ligand bonding domain with testosterone, metribolone and the designer anabolic terahydrogestrinone (THG) have shown that also more extensive Van der Waals interaction between apolar groups in the ligand and apolar side chains of the amino acids inside the receptor pocket, contribute significantly to better bonding of the ligand [1].
The possibilities for Van der Waals interactions increase with an increasing surface of the ligand molecule. The extra ethyl groups at C13 and C17 in THG do increase the surface of the molecule, which can explain the strong bonding of THG with the receptor.
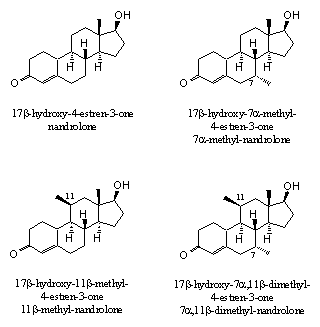 Figure 4 |
Additional prove for the effect of extra methyl or ethyl groups can be derived from the bonding affinities of the androgen receptor with nandrolone derivatives with extra methyl groups on C7, C11 or both (see Figure 4). The last compound, 17b-hydroxy-7a,11b-dimethyl-4-estren-3-one, not only showed the strongest bonding with the receptor, but also had the best separation between anabolic and androgenic effects [5].
Anabolic steroids with several extra methyl or ethyl groups are known as so called porcupine steroids. With the extended surface of these groups these molecules fix themselves better in the pocket of the receptor. It should be interesting to explore this theory a bit further and also investigate the biological properties of anabolics with additional extra methyl or ethyl groups on C 14 and C15. However, the more substituents, the more complicated the synthesis of these steroids will be, and the more expensive the anabolic will become.
The flexibility of the pocket seems to be greater then estimated before, and this may create possibilities for more porcupine steroids. This is however all pure speculation and only experiments can provide for the necessary proves. The bonding of a ligand to the receptor is rather unpredictable, sometimes similar compounds show unexplainable differences in bonding and behaviour, and sometimes markedly different compounds show similar effects.
The subtle nature of ligand-androgen receptor bonding can be illustrated also with the relative bonding affinities of testosterone, dihydrotestosterone and metribolone, which are 100, 180 and 290 respectively. From these numbers it appears that the synthetic anabolic steroid metribolone shows the highest bonding affinity with the receptor. This may be the consequence of the greater flexibility of this molecule, which may allow a better adaptation to the inside of the pocket. The greater flexibility is the result of the presence of the three double bonds in metribolone and the places of these double bonds in the molecule. It also becomes clear form the bonding affinities that the natural ligand does not always show the highest bonding affinity. There is space for improvement.
X-rays give a good impression of the ligand-androgen receptor interactions on a molecular level. This is important for the design of new selective drugs. Of course this knowledge is also essential for the design of new selective and super active anabolic steroids for instance with a better separation of anabolic and androgenic effects. Investigators also use this knowledge for the development of completely new ligands with structures that do not resemble steroids at all.
A good bonding to the receptor is not the only thing that has to be taken into account for an optimal end result. It is also important that a newly designed anabolic steroid is absorbed quickly, transported well to the place where it has to work, and not metabolized underway. The ligand-receptor complex has to initiate and maintain many interactions with other proteins that take part in the whole process. A minor change in shape of the complex may inhibit any of these necessary interactions. These effects are usually not shown in essays where just the binding affinity with the ligand bonding domain of the receptor is measured. In the next chapter we will tell more about all interactions that will bring more muscles.
[1] K. Pereira de Jesus-Tran, P-L. Cote, L. Cantin, J. Blanchet, F. Labrie en R. Breton, Protein Science (2006) 15, 987-999.
[2] J.S. Sack, K.F. Kish, C. Wang, R.M. Attar, S.E. Kiefer, Y. An, G.Y. Wu, J.E. Scheffler, M.E. Salvatie, S.R. Krystek Jr, R. Weinmann, en H.M. Einspahr, Proc. Nat. Acad. Sci. (2001) 98, 4904-4909.
[3] P.M. Matias, P. Donner, R. Coelho, M. Thomaz, C. Peixoto, S. Macedo, N. Otto, S. Joschko, P. Scholz, A. Wegg, S. Basler, M. Schafer, U. Egner en M.A. Carrondo, Journal of Biological Chemistry, (2000) 275, 26164-26171.
[4] B. He, R.T. Gample jr, A.J. Kole, A.T. Hnat, T.B. Stanley, G.An, E.L. Stewart, R.I. Kalman, J.T. Minges, and E.M. Wilson, Molecular Cell (2004) 16, 425-438.
[5] C.E. Cook and J.A. Kepler, Bioorganic and Medical Chemistry Letters (2005) 15, 1213-1216.
|
|