Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 17/1/2008
20. Selective Androgen Receptor Modulators (SARM's)
Aede de Groot, Willem Koert
The pharmaceutical industry is actively searching for successors of anabolic steroids. These new substances are called Selective Androgen Receptor Modulators or SARMís. In this chapter we will explain how SARMís look like and describe the state of the art.
The full name of anabolic steroids is in fact androgenic anabolic steroids. Scientists usually call them androgens and omit the anabolic part of the name. The reason is that the receptor that mediates the effects of androgenic anabolic steroids, is called the Androgen receptor. In Chapter 8 we have explained how this works. We have used the name anabolic steroids to emphasize the main subject of this Anabolics book.
There is only one androgen receptor and it is everywhere present in our body; in bones, muscles, liver, skin, prostate and in the central nervous system. This receptor mediates the effects of the two natural steroid hormones testosterone and dihydrotestosterone and of synthetic anabolic steroids. It does this by activating genes or other non genomic cell signaling systems.
The Leydig cells in the testes produce 90% of the testosterone in a mans body, the adrenal gland and the liver produce the other 10%. Testosterone stimulates libido and sperm production. It circulates in the blood and in muscles it acts as an anabolic steroid. The enzyme 5a-reductase converts testosterone to dihydrotestosterone in the prostate, the liver and the skin. Dihydrotestosterone is responsible for the androgenic effects, such as beard growth, body hair, acne, and in elder man for pattern baldness and enlargement of the prostate. The enzyme aromatase converts a small part (0.2%) of the testosterone into estradiol. Estradiol is essential for the growth of bones and it plays a role in libido and cognition. There are two receptors that mediate the effects of estradiol. Testosterone, dihydrotestosterone and estradiol, each in appropriate quantities, are essential for a normal functioning body.
The pharmaceutical industry has performed extensive research on synthetic anabolic steroids. This has led to medicines for hypogonadism, muscle wasting diseases, anaemia, benign prostate enlargement and prostate cancer. For women medines for mammary cancer, osteoperose and hormone therapy were developed. All this research has been only partial successful. Long lasting application of synthetic steroid hormones often showed serious side effects and liver toxicity. A lack of receptor selectively and just partial separation of anabolic and androgenic activities are the main reasons for side effects.
Anabolic steroids sometimes also fit in the steroid receptors for estrogens, progestagens, and corticosteroids. We then see the side effects that are mediated by these non-androgenic ligandĖreceptor complexes. This becomes more probable when high concentrations of anabolic steroids are present, as is the case with bodybuilders that use high doses of anabolic steroids. Well known side effects are water retention and gynecomastia.
Elder men
Anabolic steroids are not only popular with fitnessers and bodybuilders. Also elder men may benefit from the use of anabolics. The production of testosterone deminishes and the production of Sex Hormone Binding Globuline (SHBG) increases with age. Both factors are unfavourable for the availablity of testosterone for the androgen receptor. This may lead to less energy, decrease of muscle power, deminished sexual performance and sometimes to depression.
External testosterone suppletion can counteract these phenomena, but there are some drawbacks. Medics are worried about overstimulation of the prostate, and unfavourable effects on haert and blood vessels. Besides, testosterone is not orally available because of fast metabolic transformations. It can be taken parentally or a testosterone containing gel can be put on the skin. Chemists have developed a large variety of synthetic anabolic steroids, also to deal with aging problems. Many of these are described in the former chapters. However, each synthetic steroid has its own drawbacks and side effects.
Chemists, biochemists and pharmacists have been only partly successful in adapting anabolic steroids, so that they only form complexes with the androgen receptor and not with the other steroid receptors. Besides, the steroid-androgen receptor complexes ideally should only mediate the desired anabolic effects and not the tedious androgenic effects. That also has been only partially successful. Therefore, scientists and especially the pharmaceutical industry are now investigating compounds with completely different, non-steroidal, chemical structures. They try to find compounds with high selectivity for the androgen receptor, with good anabolic and no androgenic activity.
These compounds are called Selective Androgen Receptor Modulator or SARM's. They should improve muscle strength, give more energy, stimulate a better mood, libido and sexual performance, without detrimental effects on the prostate, liver, heart and blood vessels. In women they should deal with frailty and osteoperose without virilizing effects
We are all getting older and older and like to enjoy a healthy life as long as possible. Also bodybuilders and fitnessers are of course interested in good SARMís that stimulate muscle growth without riscs and tedious side effects. There is a big demand for such compounds and the pharmaceutical industry is working hard to find them. Regularly good reviews about SARMís appear in the scientific literature. [1] [2]
It is not really simple to find a good SARM. A good SARM should have the same interaction with the androgen receptor as testosterone and that should be all.
SARMís should not form complexes with the other steroid recoptors for estrogens, progestagens and corticosteroids, to avoid the side effects that are mediated by these ligand-receptor complexes. SARM's also should not bind to SHBG. In this way the SARM remains available for the androgen receptor and can be applied in a lower dose. Also possible non-genomic effects of SHBG-SARM complexes will not be mediated.
SARMís should not inhibit the enzyme aromatase. The body needs the small quantity of estradiol that is also produced by men, for bone formation and cognition.
A good SARM should not or as little as possible, interfere with the natural production of testosterone and sperm. The production of testosterone in the Leydig cells in the testes is under control of the so called Hypothalamus-Pituary-Testes Axis (HPT-axis). This process starts with nerve stimulation of the hypothalamus to secrete the Gonadotropin Releasing Hormone (GnRH). This is a small peptide hormone (see Chapter 3), which stimulates the pituary to release two glyco-peptide hormones, the Luteinizing Hormone (LH) and the Folikel Stimulating Hormone (FSH) (see Figure 1).
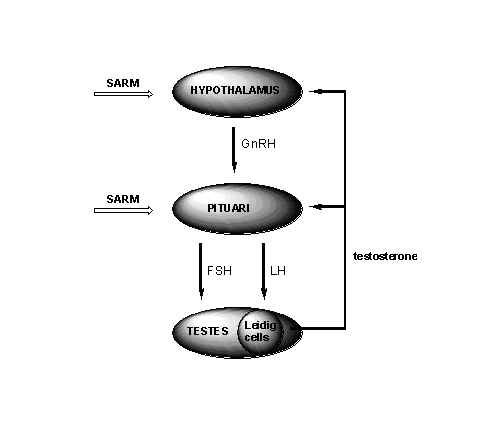
Figure 1
LH then stimulates the Leydig cells in the testes to produce testosterone and the concentration of testosterone in the blood increases. This higher concentration of testosterone on its turn will inhibit the further production of GnHR and LH. The testosterone in the blood is metabolised in the liver and removed from the body. This results in a lowering of the concentration of testosterone in the blood. This lower concentration will decrease the inhibition of the hypothalamus and the pituary, and the whole process restarts. This feedback regulation of the testosterone production prevents large fluctuations of its concentration in the blood.
Synthetic anabolic steroids in high concentrations also inhibit the release of GnRH, LH and FSH and in this way the bodyís own production of testosterone and sperm is stopped. The testes have nothing to do and shrink. When the use or abuse of anabolics is stopped the production of testosterone and sperm recovers but this may take some time.
A good SARM should not inhibit the secretion of GnRH, LH and FSH completely and that is a problem. In its interactions with receptors the SARM resembles testosterone. In the androgen receptor in the muscles this is a desirable interaction, but the interaction with the receptors in the hypothalamus and the pituari should not lead to inhibition of the release of GnRH, LH and FSH. The opposite is desired for SARM's that are developed for male contraception. These SARM's are ment to suppress or stop the sperm production.
It will not necessarily lead to problems when the bodyís own production of testosterone will be down to a low level due to the application of a SARM. The SARM itself will take over the anabolic function of testosterone. When there is very little testosterone also the production of dihydrotestosterone will be low. That is an advantage because then also the androgenic effects of dihydrotestosterone will be prevented. The body does need the small quantity of estradiol, but in most cases there will be enough testosterone produced, to take care of that.
The SARM should be orally available, and preferrably should not be taken more often then once per day. This means that metabolic transformations of the SARM should not be too fast. The SARM itself and its metabolites should not be toxic.
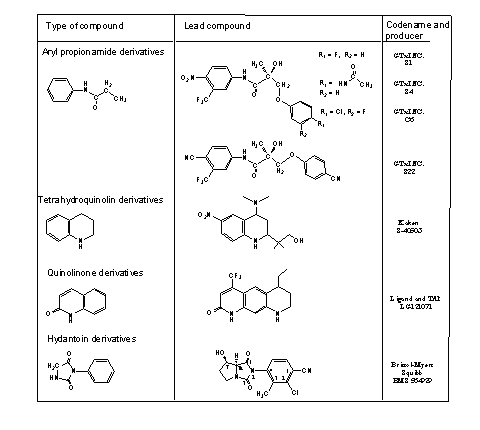
The perfect SARM has to fulfill all these conditions. But just as with anabolic steroids, one SARM does this in a better and different way then the other. Untill now four groups of SARM's with promising properties have been published. These four groups are mentioned in Tabel 1. All these SARM's are androgen receptor agonists with good bonding to the receptor. The SARM-androgen receptor complexes show good anabolic activities in animal tests. They are not yet on the market but they are in the pipe line. S1, S4, C6 and BMS 564929 are presently under clinical trial.
Table 1
(Click for enlargement)
It is clear that the structural formulas of these SARM's do not at all resemble those of anabolic steroids. Nevertheless they give stable complexes with the androgen receptor. Also in these compounds there are functional groups that can form hydrogen bonds and take part in Van der Waals interactions with the side chains of the amino acids that line the inside of the bonding pocket of the androgen receptor. These interactions together lead to bonding of the ligand.
After bonding of the ligand the ligand-androgen receptor complex changes its shape, which induces tissue specifix processes. These processes are in the skin and the prostate different from those in muscles. One of the reasons is that in each tissue different enzymes and cofactors are present. These are collected by the ligand-androgen receptor complex on the DNA and together they transactivate the target genes for proteine synthesis (see Chapter 8, figure 2). The SARM has to be more selective in these processes then the usual synthetic anabolic steroids. The SARM-androgen receptor complex has to initiate just the anabolic effects in the muscles and, for instance, not the androgenic effects in the prostate. In principle this should be possible.
From crystal structure determinations of complexes of steroidal ligands with the ligand bonding domain (LBD) of the androgen receptor we know how these complexes look like on a molecular level, and which interactions play important roles (see Chapter 7). Also the crystal structures of a number of SARM-LBD complexes have been determined. From these structures, scientists can see where the SARM-LBD structures differ from those of the natural and synthetic anabolic steroids.[3] [4] Chemists use this information to optimalize the structure of the SARM and to explain the selectivity of its effects.
The SARM agonists [5] from Table 1 have originated from knowledge about similar types of compounds which are already applied as anti-androgens and as androgen antagonists. Agonists and antagonists [6] for the androgen receptor and anti-androgens [7] have in common that they all bind with the receptor. However, all three types of compounds do this in a slightly different way, so that also the follow up is different. The chemical structure of agonists and antagonists for the same receptor sometimes resemble eachother rather closely.
We will have a closer look to the SARM's that nowadays are under clinical trial. Some members of the group of aryl propionamides are already on the market as anti-androgens. Based on their structures, the SARM's S1, S4, C6 and later S22 are developed. They show good anabolic activity and have only a partly androgenic effect on the prostate. They do suppress the secretion of LH and FSH. Especially C6. shows this effect, and pharmacists consider application of this compound in male contraception.
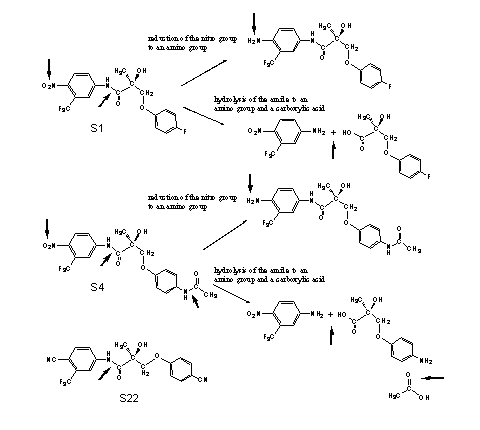
The propionamide SARM's are orally available, but their halflife time is approximately three hours and that is relatively short. The main metabolic reactions are reduction of the nitro group and hydrolysis of the amides (see Scheme 1). In the second generation compound S22, the nitro group and the halogen atoms or amide group are replaced by cyano groups. These are not metabolically reduced and the halflife time of S22 is about six hours.
Scheme 1
(Click for enlargement)
Bristol Meyers Squibb has developed a hydantoine type SARM named (7R,7aS) 2-chloro-4 (7-hydroxy-1,3-dioxotetrahydropyrrolo[1,2c]imidazol-2-yl) 3-methyl-benzonitrile, with the codename BMS 564929. [4] The synthesis of BMS 564929 is rather complicated. The right and the left part of the molecule each take five steps. Another three steps are necessary to put the two halves together and to finish the molecule.
BMS 564929 binds well to the androgen receptor and not to the other steroid receptors. BMS 564929 has no interactions with SHBG and it does not inhibit aromatase. Tests with rats show a better anabolic activity then testosterone propionate at the same dose, and a low androgenic activity. At higher doses BMS 564929 does inhibit the hypothalamus and\or the pituary and in this way lowers the secretion of LH and FSH.
An X-ray of the complex of BMS 564929 with the LBD of the androgen receptor shows some differences between BMS 564929 and dihydrotestosterone in their interaction with the LBD of the receptor. These differences seem to be large enough to enable mediation of the anabolic effect in the muscles and not the androgenic effect in the prostate. It is not yet completely clear how to explain all the differences.
BMS 564929 is orally available and metabolic transformations are slower then in the proionamide SARM's. The halflife time varies from eight to fourteen hours, and a low dose is possible.
Critical reviews and comments in the scientific literature generally are positive about SARM's. [8] [9] It is however also clear that more research is necessary.
The anabolic steroids have ended up ultimately in the grey and black zone of doping and designer supplements. Maybe that will happen also with SARM's in the future. There are however a few differences between SARM's and anabolic steroids.
A SARM with high anabolic activity that is attractive for sportsmen and bodybuilders, still has to be found. When this will happen, the makers of designer SARM's and illegal supplements have a problem. It is not that easy to change the structure of a SARM a bit, to enhance its anabolic activity or to circumvent patents. Any minor change in its chemical structure may change an agonist into an antagonist. This last remark should be a warning for chemical sportsmen that are offered designer SARM's. They just as well may have an opposite effect.
[1] Gao W.; Kim J. and Dalton J.T. Pharmaceutical Research (2006) 23, 1641-1658.
[2] Gao W.and Dalton J.W. Drug Discovery Today (2007) 12, 241-248.
[3] Bohl C.E.; Miller D.D.; Chen J.; Bell C.E. and Dalton J.T. J. Biol. Chem. (2005) 280, 37747-37754.
[4] Ostrovski J.; Kuhns J.E.; Lupisella J.A.; Manfredi M.C.; Beehler B.C.; Krystek S.R.; Bi Y.; Sun C.; Seethala R.; Golla B.; Sleph P.G.; Fura A.; An Y.; Kish K.F.; Sack J.S.; Mookhtiar K.A.; Grover G.J. and Hamann L.G. Endocrinology (2007) 148, 4-12.
[5] An agonist for the androgen receptor is a synthetic compound that binds with the receptor. The complex gives the same natural follow up as the complexes of the natural ligands testosterone and dihydrotestosterone.
[6] An antagonist for the androgen receptor is a synthetic compound that does bind to the receptor, but the complex does not give any of the natural follow up processes.
[7] An anti-androgen is a synthetic compound that competes with the natural ligands for binding with the androgen receptor. The complex does not give the natural follow up processes.
[8] Brown T.R. Endocrinology (2004) 145, 5417-5419.
[9] Wilson E.M. Endocrinology (2007) 148, 1-3.
|
|