Het Anabolenboek
Willem Koert
Aede de Groot
Minsk, 23/8/2007
16. Esters, Enol-esters, Carbonate-esters and Carbamates
Aede de Groot, Willem Koert
Next to the prohormones there is a second group of steroids which enter the body as inactive compounds. In the body they are transformed into active anabolic steroids by enzymes. This group consists in the first place of esters of anabolic steroids but also acetals, enol ethers and normal ethers have appeared on the market. We will treat these compounds as a separate group. We call them hormone derivatives.
The difference between prohormones and hormone derivatives is the place of the enzymatic transformation in the steroid. In a prohormone the transformation into the real hormone takes place by an enzymatic reaction on the steroid skeleton. In a hormone derivative the transformation into the real hormone takes place by an (enzymatic) reaction at one of the substituents. In anabolic steroids mostly the hydroxyl group at C17 is derivatized, sometimes this is the case with the hydroxyl group or the carbonyl group at C3, and occasionally the derivative is at another place.
We will look at the characteristic chemical properties of hormone derivatives to understand in which way they are converted to active anabolic steroids. There are two ways to do this:
- The derivative is converted to the real hormone by an enzyme. For instance an esterase
can hydrolyze esters like testosterone propionate or nandrolone decanoate into the free steroids
testosterone or nandrolone.
- The derivative is converted to the real hormone by a normal chemical reaction. For instance gastric
acid can hydrolyze an acetal or an enol ether.
In this chapter we will have a closer look at esters of anabolic steroids, in the next chapter the other derivatives will be treated.
Chemical properties of carboxylic acids and esters
Thousands of carboxylic acids and thousands of alcohols are known and they can react to esters in all possible ways. A large variety of esters occurs in Nature. Because of all these possibilities, it will be good to pay attention to the nomenclature and chemistry of carboxylic acids, their salts and their esters.
The carboxyl group is the characteristic functional group in carboxylic acids (see Scheme 1). The carboxyl group can be considered as a combination of a hydroxyl group and a carbonyl group at the same C-atom. For this reason they can interact with each other and this interaction gives the carboxyl group its own characteristic reaction possibilities.
One of these characteristic chemical properties of the carboxyl group is its acidity, and from this the name carboxylic acid originates. When a carboxylic acid is dissolved in water, it dissociates partly in a carboxylate and a proton (H+), which is bound to water (see Scheme 1). Ordinary vinegar is a 3-4% solution of acetic acid in water.
The proton of the carboxyl group can be replaced by a metal ion like sodium or potassium (Na+ or K+) to give a sodium or potassium salt of the carboxylic acid. The H-atom also can be replaced by a methyl or ethyl group or by some other substituted C-atom, and then it becomes an ester. In both cases the suffix in the name carboxylic changes into carboxylate (see Scheme 1)
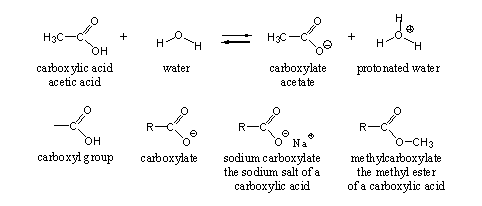
Scheme 1
In Table 1 the names and suffixes of the first ten linear carboxylic acids, esters and salts are presented. Linear means that the carbon chain in not branched. In Figure 1 we see that esters of branched chain or aromatic carboxylic acids occur also.

Table 2 mentions a number of fatty acids, that occur in esters of anabolic steroids.

The reaction of a carboxylic acid with an alcohol gives an ester and water. The reaction is an equilibrium, an ester can be hydrolyzed again with water to a carboxylic acid and an alcohol. The body uses this last reaction to set free the active anabolic steroid from its ester. This reaction is catalyzed by esterases.
In scheme 2, the formation and hydrolysis of the ester of acetic acid and ethanol is shown. In the same scheme the hydrolysis of the ester nandrolone decanoate is shown. We see from these examples that the hydroxyl group can be part of a simple molecule like ethanol or of the more complicated molecule nandrolone. Likewise the carboxylic acid can be simple like acetic acid or it can have a longer carbon chain as in decanoic acid. In Figures 1 and 4 carboxylic acids can with more complicated structures are shown.
Esterases occur in blood and are not very selective. They accept different substrates and can hydrolyze many different esters of steroids.
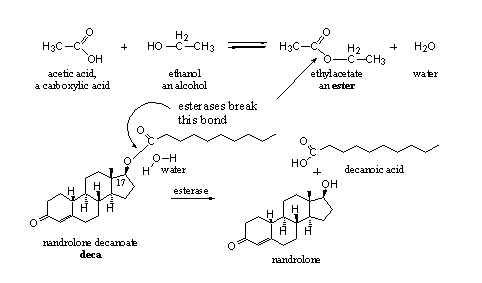
Scheme 2
Steroid esters
In Figure 1 we have collected the esters of testosterone that have appeared on the market.
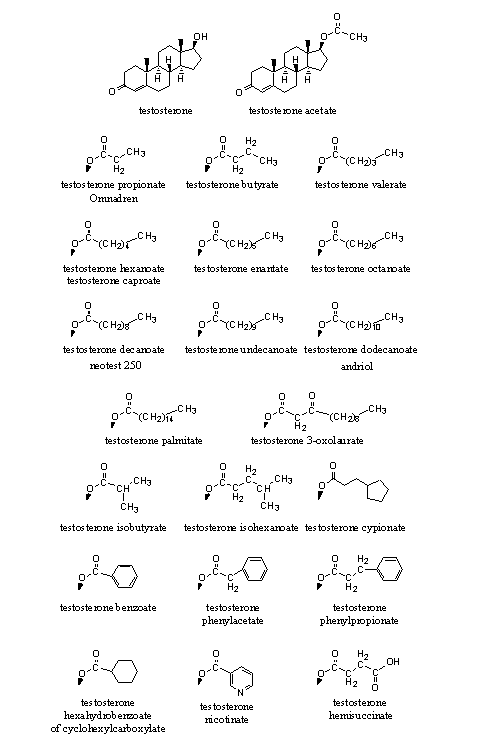
Figure 1
In Figure 2 the esters of nandrolone are collected that have been marketed. These esters resemble those of testosterone in figure 1. An interesting detail: We have found 13 producers of these nandrolone esters and nine of them are located in China, one in India, one in Italy, one in Hungary and one in The Netherlands.
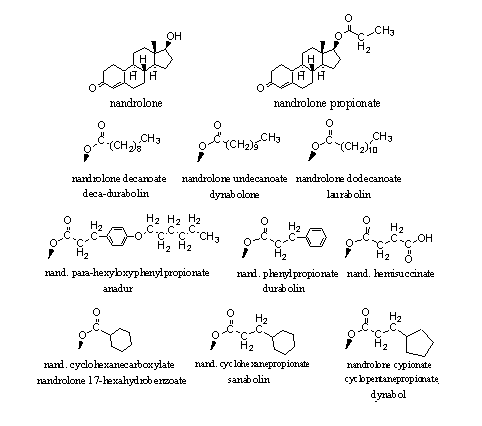
Figure 2
Not only testosterone and nandrolone are for sale as esters, also esters of boldenone, trenbolone and other steroids are on the market. In principle there are few restrictions. Examples are shown in Figure 3.
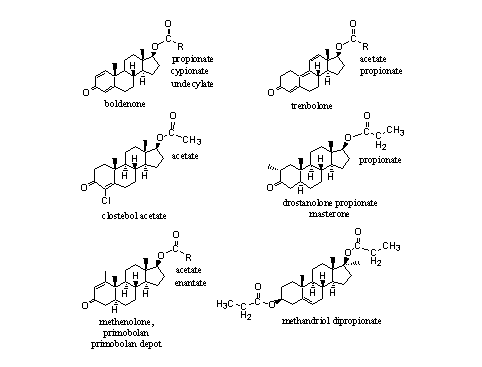
Figure 3
Esters of 17a-methyl steroids are known, but they have not appeared often on the market. Methandriol dipropionate is one of the few examples (see Figure 3, right bottom corner). It is not necessary to convert 17-methyl steroids into esters because these steroids are orally available themselves and they do not metabolize fast. Also the tertiairy hydroxyl group (see Chapter12, Scheme 3) in 17-methyl steroids is more sterically hindered and therefore less easily transformed into an ester. Esterases also hydrolyse these esters less easily.
The structure of the ester determines the velocity with which it will be hydrolyzed by esterases. More steric hindrance usually causes slower hydrolysis. Other conditions such as lipid solubility, transport in the body, vulnerability for metabolic transformations and patent possibilities determine which combination of steroid and carboxylic acid will be marketed as ester.
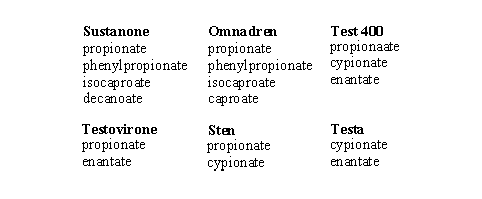
In anabolic preparations also mixtures of slower and faster hydrolysing esters are found, this to affect a direct and a longer lasting anabolic effect. In Table 3 some ester mixtures are mentioned, which are marketed under their own tradename.
In patents and in the scientific literature many esters are mentioned, which for some reason never have reached the market. Their anabolic activity has been established but not exploited. Some of these esters are collected in Figure 4 to give an impression of their sometimes exotic structures.
The compounds on the top row in Figure 4 have been synthesized to investigate the role of steric hindrance on their hydrolysis velocity [1] [2]. The hydrolysis is slow in these highly hindered esters, but does occur. The esters have a long lasting anabolic effect.
The structure in the middle on the left is a bit strange [3]. The C17 hydroxyl group first has formed a so called hemi acetal with chloral (trichloroacetaldehyde). Next the resulting hydroxyl groep has been converted into an ester.
Also the other esters in Figure 4 are formed from somewhat strange carboxylic acids [4] [5] [6]. The anabolic activity of the esters from Figure 4 is good, with mostly a better separation of anabolic and androgenic effects then the parent steroids.
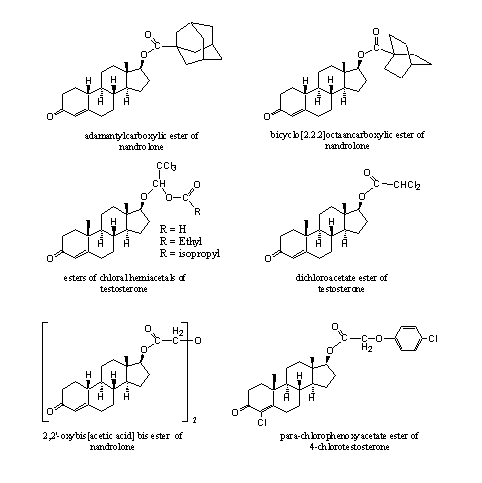
Figure 4
Enol ester and dienol ester derivatives
Many esters on the market have an ester group at C17, but it is of course also possible to attach an ester at C3. However, it would be better when after hydrolysis of an ester at C3, a carbonyl group should remain instead of a hydroxyl group. This is possible with an enol ester or a diŽnol ester derivative of the C3 carbonyl group.
In Chapter 14 we have already explained that an enol consists of a double bond (-en) with an attached hydroxyl group (-ol). A dienol consists of two double bonds next to each other with a hydroxyl group attached to one of them. When the hydroxyl group is converted to an ester an enol or dienol ester is obtained. In scheme 3 a dienol ester of testosterone is described. The carbonyl group at C3 enolizes in the direction of ring B and the D4-double bond shifts one place to the D5-position.
Practically all carboxylic acids can be used to make a (di)enol ester but an acetate is the easiest to synthesize. Many (di)enol acetates of steroids are known in the literature. After injection of these derivatives the esters are slowly hydrolized by esterases to set free the (di)enol. After that the 4-en-3-one structural element of testosterone is reconstructed in a spotanuous equilibrium reaction.
It is possible that in the synthese of a (di)enol ester also the hydroxyl group at C17 is transformed in an ester (acetate). This can be prevented by first protecting this hydroxyl group, followed by deprotection after formation of the (di)enol ester. However this costs two reaction steps which will make the endproduct more expensive. Besides, it is not a drawback when also the hydroxyl group at C17 will be esterified because esterases can also hydrolyse this ester (see Scheme 3).
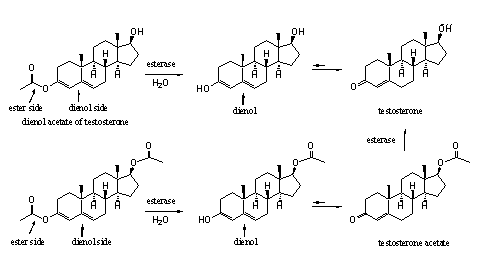
Scheme 3
The anabolic activities of the dienol esters in Figure 5 has been demonstrated and patented already in the fifties and sixties of the former century. The 3-dienol acetates of 7a-methyl-testosterone-17-acetate, the 7a,17a-dimethyl derivative and its acetate are described in a patent of Upjohn as useful compounds with anabolic, androgenic, anti estrogenic, and hypo cholesteremic activity (see Figure 5) [7].
Several dienol esters of nandrolone were patented already in 1956 by the same company. They were claimed to have a high and elongated anabolic activity with a good separation between anabolic and androgenic effects [8] (see Figure 5).
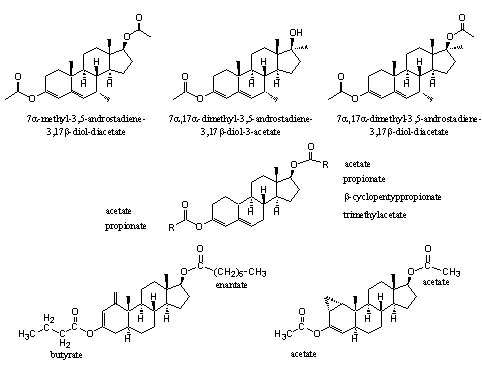
Figure 5
In 1965 the Schering company patented the peculiar dienol and enol esters at the bottom of Figure 5. The combination of the 3-butyrate-17-enantate of 1-methylene-5a-androst-2-en-3-enol (bottom left) and 17b-hydroxy-1-methyl-5a-androst-1-en-3-one provides for a quick and longlasting anabolic effect and a low androgenic activity in rats [9].
The 1a,2a-methylene-3-enol-diacetate bottom right showed a strong anabolic effect and only little androgenic activity [10].
Carbonates and carbamates
Carbonates are esters of carbonic acid. Carbonic acid itself is an unstable acid, which quickly decomposes in carbondioxide and water. Both hydroxyl groups in carbonic acid can form esters with the same or with different alcohols.
Esters of carbonic acid are a bit more stable then normal esters, but they are only stable when both alcohol groups are esterified. As soon as one of the esters is hydrolysed to an hydroxyl group, the remaining half ester will decompose spontanuously into the second alcohol and carbondioxide (see Scheme 4).
Trenbolone cyclohexylmethyl carbonate is for sale on the market, the second alcohol is also named hexahydrobenzyl. Esterases may hydrolyse carbonates on both sides. In trenbolone cyclohexylmethyl carbonate this probably will happen at the least hindered cyclohexylmethyl side. After spontanuous decomposition of the remaining half ester, trenbolone will be set free.
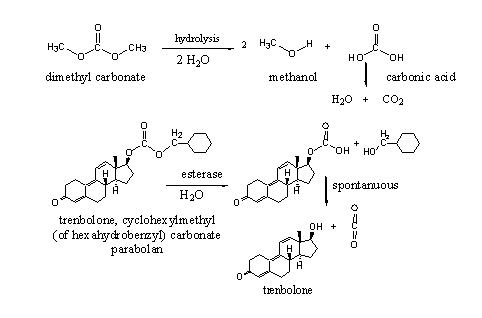
Scheme 4
A substantial number of steroidal carbonates is known in patents and from literature and some of them are shown in Figure 6. The cyclohexylmethyl [11] [12] and the adamantyl [13] carbonates of testosterone and nandrolone are known. They have been synthesized and tested with the same intentions as the corresponding normal esters.
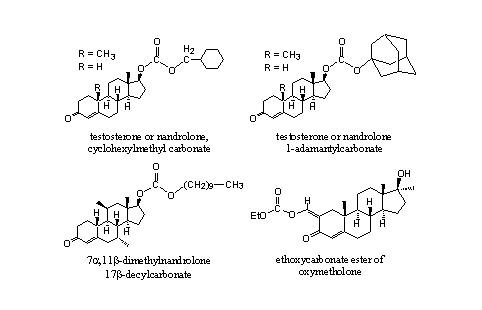
Figure 6
Also carbonates of other steroids are known. The 17a-decylcarbonate of 7a,11b-dimethylnandrolone has a five times larger anabolic activity then methyltestosterone. [14] The ethylcarbonaat of oxymetholone shows an activity that is comparable with that of oxymetholone itself. It is assumed that first hydrolysis will take place to oxymetholone itself, which is considered to be the active component. [15]
A second kind of carbonic acid derivative, which has been investigated for anabolic activity, are steroidal carbamates. Carbamates are mixed ester-amides of carbonic acid. (see Figure 7) Their chemical behaviour is similar to that of carbonates.
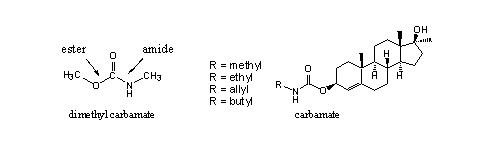
Figure 7
A limited number of steroidal carbamates with useful anabolic activity is known [16]. The investigators assume that the carbamate itself is the active compound. No hydrolysis to the free steroid should be necessary. Enzymes which are capable to hydrolyse carbamates have been found in rats, but it is not known whether they are also present in humans.
[1] Rapala R.T., Kraay R.J., Gerzon K. J. Medicinal Chem. (1965) 8 580-583.
[2] Schibmer R.M., Dorfman R.I., Rooks W.H. J. Medicinal Chem. (1970) 13 952-956.
[3] Borrevang P. Danish patent DE 1114488 19611005.
[4] Kincl F.A., Dorfman R.I. Steroids (1963) 3 109-122.
[5] Van der Vies, Ger. Offen. DE 75-2553997.
[6] Doerner G., Kleinert E. Acta Biologica et Medica Germanica (1963) 11 77-85.
[7] Upjohn, Dutch patent, NL 6604702 19661010.
[8] Upjohn, English patent, GB 755129 19560815.
[9] Mueller H., Neumann F., Wiechert E. (Schering), Artzneimittel-Forschung (1966) 16, 1515-1518.
[10] Wiechert R. (Schering) German patent DE 1187611 196550225.
[11] Roussel-UCLAF patent BE 615644.
[12] Roussel-UCLAF patent FR M2444 19640504.
[13] Boswell G.A. South Afrikan patent ZA 6706588 19680308.
[14] Blye R.P., Kim H.K. Patent No WO 2006083618.
[15] Evans D.D., Palmer P.J. Steroids (1965) 5 441-450.
[16] Fahrenholtz K.E., Bloomfield N.J. US patent 3 787 453.
|
|