Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 23/4/2007
13. Chemical Tricks to Prevent Undesired Transformations of Anabolic Steroids
Aede de Groot, Willem Koert
The possible metabolic transformations of injected or oral anabolic steroids have been discussed already in chapter 10 and 11. In the previous chapter 12 we have shown that small chemical modifications of the steroid molecule may slow down or prevent these enzymatic transformations. Many synthetic anabolics resemble the natural steroid hormones quite closely and for that reason they also can be accepted by the corresponding enzymes as a substrate. We now will show for all groups of enzymes that are involved in the metabolism of anabolic steroids how their adverse effects may be prevented.
Cytochrome P450-enzymes
Cytochrome P450 enzymes together with oxygen, introduce hydroxyl groups into the steroid molecule. This usually is the first step that is necessary for excretion of the steroid from the body. Other enzymes attach a glucuronide or a sulphate moiety to these hydroxyl groups and in this way the polarity of the whole molecule is increased sufficiently to make it soluable in water. Now the kidnies can excrete the steroid together with the urine.
The introduction of hydroxyl groups at other places then necessary for the biosynthesis of the natural steroid hormones, has been notices for C6 and C16 and sometimes for C12, C18 and C19. The oxidation of C6 is fairly obvious because this position is more reactive than the other places in the molecule, because it is located next to the double bond.
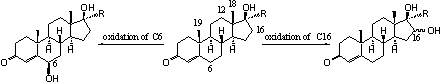
Scheme 1
Reactions that are catalysed by cytochrome P450 enzymes are dependent on the structure of the whole steroid. When the most obvious places are occupied or inaccessible for reactions sometimes other places are oxidized. Some reactions which are in principle possible do not occur because the steroid has been removed already from the cell by conversion to a glucuronide or a sulphate. This is a realistic possibility when the steroid already contains one or more hydroxyl groups.
It is not realistic to try to block cytochrome P450 enzymes. These enzymes are so essential for the general metabolism of the body that it is not sensible to attempt such actions.
Oxido-reductase-enzymes at C17
The carbonyl group at C3 and the hydroxyl group at C17 are essential for a good interaction of the steroid ligand with the androgen receptor and each enzymatic transformation of these groups is detrimental. The example in chapter 12 shows that a 17a-methyl group can prevent the oxidation of the C17b-hydroxyl group efficiently. For a long time it has been thought that even bigger groups at C17 would be a handicap for good interaction with the receptor, but calculations and the experience with THG have shown that this is not necessarily the case.
The introduction of a 17a-substituent has become a general recipe to protect the 17b-hydroxyl group for oxidation and to improve the anabolic properties of the steroid. It also has been mentioned that the 17a-substituted anabolics are a serious risk for the liver and for that reason they should not be used before serious testing has shown their safety.
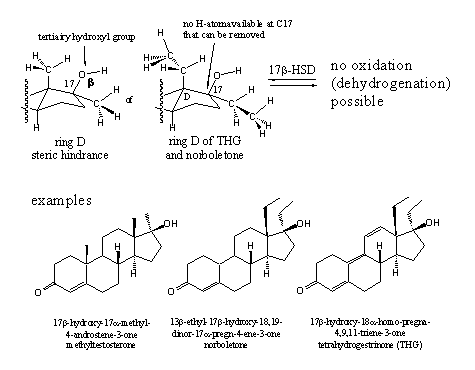
Scheme 2
Oxido-reductase-enzymes at C3
It is more difficult to prevent the reduction of the C3 carbonyl group to an a- or b-hydroxyl group. In chapter 2 is shown that a C=C double bond is flat and the same is true for a C=O double bond. This means that a carbonyl group is easily accessible from the upper and from the bottom side for reaction. For reduction H-atoms have to be added and not abstracted as at C17 and for this addition there is plenty of space. Since all possible four bonds of C3 are already in use for binding the C-atoms in the ring and the O-atom, there is also no place left for additional groups, so the steric hindrance at C3 can not be increased.
More steric hindrance for reduction can only be brought into the molecule on the C-atoms around the C3 carbonyl group, so on C2 or C4 and this has been carried out (see Scheme 3). A methyl group has been introduced at C2 in drostanolone and in stenbolone. In clostebol the chlorine atom at C4 takes care for more steric hindrance. In stenbolone the additional D1-double bond and in clostebol the D4-double bond provide for extra stability of the C3 carbonyl group against reduction.
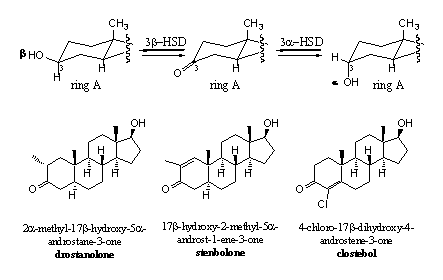
Scheme 3
A second possibility is to increase the stability of the carbonyl group itself and make it more resistant to reduction. This can be achieved by making the carbonyl group part of a conjugated system, this is a system with alternating double and single bonds (C=C-C=O). We do find these conjugated systems in many anabolic steroids, for instance between C1 and C2 (D1), between C4 and C5 (D4) or both and also more extensive conjugated systems as in THG, are applied (see Scheme 3 and Figure 1).
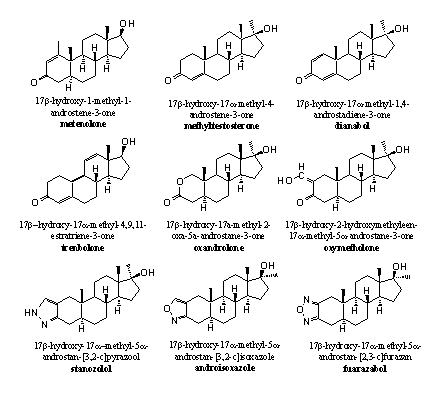
Figure 1
A third possibility is to construct a completely different functional group at the place of C3 or around it, with or without a carbonyl group. Examples are oxandrolone and oxymetholone (see Figure 1).
In oxandrolone the carbonyl group is part of a cyclic ester. Chemists call such a group a lactone. Lactones are more stable for reduction then a carbonyl group. The O-atom in the ring takes care of a stabilising interaction with the carbonyl group.
In oxymetholone the carbonyl Group is part of a conjugated system but now with a so called exocyclic double bond, this is a double bond outside the ring. The hydroxyl group at this double bond provides for an additional stabilising interaction.
In stanozolol, androisoxazole and furazabol, the carbonyl Group is replaced by a small ring. This heterocyclic ring is aromatic and therefore extra stable. The indication heterocyclic means that one or more of the C-atoms in the ring are replaced by other atoms, as in these cases by N- or O-atoms. The lower N-atom in these rings is a hydrogen bond acceptor and probably takes care of this interaction instead of the carbonyl group.
The double bonds and the additional rings also cause changes in the shape of the steroid ligand. This can be advantageous or disadvantageous for the binding of the ligand to the androgen receptor. The best case is of course that a diminished interaction with the enzyme goes together with a better binding with the androgen receptor. This is not excluded beforehand because the enzyme and the receptor are two different proteins.
Reductases
Isomerases that catalyze the shift of the C5-C6 (D5)-double bond to the C4-C5 (D4)-position participate in the biosynthesis of steroids. This shift is necessary for an easy reduction of this double bond. The reduction of the D4-double bond is easier when it is conjugated with and activated by the C3 carbonyl group. It has been remarked before that this conjugation stabilizes the carbonyl group for reduction and that is true. For the double bond it is exactly the opposite, it can be attacked by reducing agents easier when it is conjugate with a carbonyl group. The reason for this is known and originates from the type of reduction. We will not explain this here.
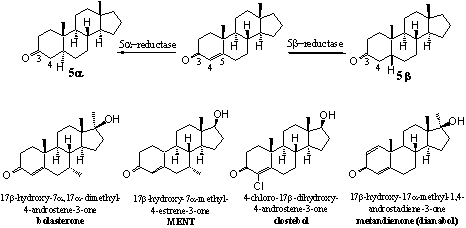
Scheme 4
The reduction of the D4-double bond can take place from the upper side of the molecule, which will lead to a 5b-steroid, or from the bottom side of the molecule which gives the 5a-steroid. The introduction of extra substituents like a 7a-methyl group in bolasterone and MENT may hamper the action of 5AR and prevent reduction. Methyl groups at the bottom side of the molecule hinder the approach of the co-enzyme that has to deliver the H-atom there.
Also the introduction of a Cl-atom at C4, as in clostebol, makes the reduction of the D4 double bond more difficult. This is not only the result of steric hindrance but the Cl-atom also has a stabilising interaction with the double bond.
The introduction of a D1-double bond, as in dianabol, is also detrimental for reduction of the D4-double bond. The reason for this effect is not really clear. It may be that the change in shape of the molecule may lead to a misfit of the molecule in the reducing enzyme 5AR. An other reason may be that the activating effect of the carbonyl group now has to be divided over two double bonds. For this reason the activation may be insufficient for reduction of the D4-double bond.
The reduction of the D4-double bond in testosterone, catalysed by the enzyme 5AR, leads to dihydrotestosterone, which has an undesired androgenic activity. When this reduction is catalysed by a 5b-redutase it will give the inactive 5b-steroid. Also the further reduction of the C3 carbonyl group to a hydroxyl group is easier after reduction of the D4- double bond and also this is a disadvantage.
It is not always predictable in synthetic anabolic steroids, whether reduction of the D4-double bond will be advantageous or disadvantageous for the activity. There are several anabolic steroids known in which no D4-double bond is present. Thus what counts for testosterone and dihydrotestosterone is not necessarily valid for other anabolic steroids. It has been remarked before that every change in the structure of anabolic steroids has its own consequences. If you like to know these you have to test the molecule first extensively.
Glucuronidases and sulfatases
The enzymatic reactions that are catalysed by sulfatases and glucuronidases have been described already in van chapter 10 in Schemes 11 and 12. These reactions involve especially the hydroxyl groups at C3 and C17. These hydroxyl groups are transformed in more polar sulfates or glucuronides so that they can be excreted better. These are in principle undesired reactions because they diminish the concentration of active compound in the body.
Both reactions are usually retarded or inhibited by steric hindrance. The steric hindrance can be increased by the introduction of bulky groups in the neighbourhood of the hydroxyl groups that have to react. The presence of a 17a-methyl or -ethyl group will hinder sulfate or glucuronide formation of the 17b-hydroxy group.
Only hydroxyl groups can be converted into glucuronides or sulfates. A carbonyl group can not be transformed into these derivatives. This carbonyl group has to be reduced first to a hydroxyl group and only after that a reaction becomes possible. This means that we have to diminish the chance that reduction of the C3 carbonyl group will take place. We have seen in Scheme 4 and Figure 1 how this can be achieved.
Esterases
Esterases mostly catalyse reactions of anabolic steroids that are desired. In anabolic steroids no ester groups are present that can be transformed so there is also no problem. Anabolic steroids are often administered as an ester, a simple example is decadurabolin, which is an ester of nandrolon. Esterases will hydrolyse the ester slowly and in this way the active anabolic steroid is released slowly and will have a longer lasting effect.
|
|