Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 13/4/2007
12. How to Prevent Undesired Metabolic Conversions of Anabolic Steroids
Aede de Groot, Willem Koert
Enzymatic conversions of injected or orally taken anabolic steroids have been described in Chapter 10. In Chapter 11 we have seen how enzymes catalyse the biosynthesis and the metabolism of testosterone and dihydrotestosterone. The most important reaction sites and the relevant types of enzymes are summarized in Figure 1.
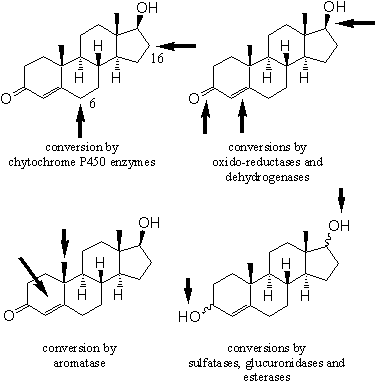
Figure 1
We now first will discuss how to prevent undesired enzymatic transformations of anabolic steroids. After that we will see in chapters 13 and 14 how this actually has been carried out for the different types of transformations. Many synthetic anabolic steroids resemble the naturally occurring steroids in our body. For this reason these enzymes often also can transform the synthetic anabolics just as well. However chemists can change the structure of synthetic anabolics a bit so that the enzymes will have difficulties to do their job.
For a better understanding it is necessary to explain again the differences between enzymes and receptors. An enzyme binds a substrate to stimulate a reaction to another compound. A receptor binds a ligand to change shape together, no reaction has to take place, the ligand leaves the receptor unchanged after the interaction.
Anabolic steroids preferably have to work for a longer period and fast metabolic transformations by enzymes have to be prevented. The interaction of the anabolic steroid with the androgen receptor must remain possible, which means that the changes in the anabolic steroid have to be relatively small because it still has to fit in the pocket of the receptor. At the same time the changes have to be so that transformation by the enzymes should become more difficult or impossible.
This problem can be tackled in two ways.
- The substrate has to become chemically incapable to undergo the enzymaticreaction. - Chemical transformations can be inhibited or delayed by steric hindrance.
A nice example to illustrate both points is the introduction of a 17a-methyl group in the steroid molecule. This extra methyl group prevents oxidation of the desired 17b-hydroxy group to an undesired C17 carbonyl group. At the same time it hampers the formation of glucuronates or sulfates.
A methyl group is small enough to make a good fit of the anabolic steroid in the pocket of the androgen receptor still possible. A methyl group is also small enough for a good fit of the substrate in the reaction pocket of the enzyme but nevertheless the reaction will not take place. The reason is a very basic chemical fact. When the 17a-H-atom is replaced by a methyl group no dehydrogenation can take place anymore. There is no H-atom attached anymore at C17 which can be removed.
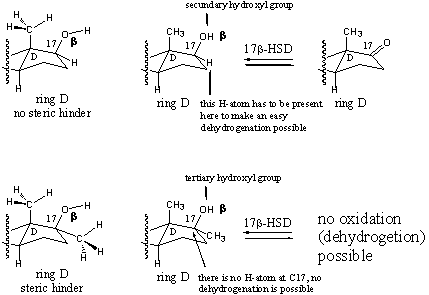
Scheme 2
In more chemical terms we can say that the C17-hydroxyl group in testosterone is a secundary hydroxyl group. This can be oxidized in a normal way to a carbonyl group by a dehydrogenase. A H-atom is removed from C17 and a H-atom is removed from the O-atom of the hydroxyl group under formation of a carbonyl group. When the 17a-H-atom is replaced by a 17a-methyl group as in methyl testosterone then the secondary hydroxyl group is transformed into a tertiary hydroxyl group and such a hydroxyl groups can not be oxidized in a simple way. There is no 17-H-atom anymore that can be removed.
Oxidation of a tertiary hydroxyl group can only take place under forced conditions after breaking of one of the C-C bonds at the hydroxyl bearing C-atom and that is a complicated process. In Scheme 2 this is explained again for simple alcohols.
There are three types of hydroxyl groups (alcohols) primary, secondary and tertiary. These are defined by the number of bonds to other C-atoms of the C-atom that bears the hydroxyl group, as is indicated in Scheme 2. During the dehydrogenation, H-atoms are removed from the hydroxyl group and from the C-atom to which the hydroxyl group is attached, these H-atom are printed bold in Scheme 2.
These two H-atoms have to be removed simultaneously. In this way a primary hydroxyl group as in ethanol is dehydrogenated easily to aceet aldehyde. After consumption of alcoholic drinks, this reaction usually already takes place partly in the stomach, especially when it is filled with food. That is why you will not get drunk so quickly when you eat while you drink. This in contrast to drinking on an empty stomach, in that case the ethanol is absorbed very quickly by the blood and consequently you will get drunk rather fast.
The secondary alcohol can be transformed easily in a similar way by dehydrogenation to acetone, but in the tertiary alcohol there is no H-atom attached anymore to the C-atom that bears the hydroxyl group and that makes an easy dehydrogenation by a dehydrogenase impossible.
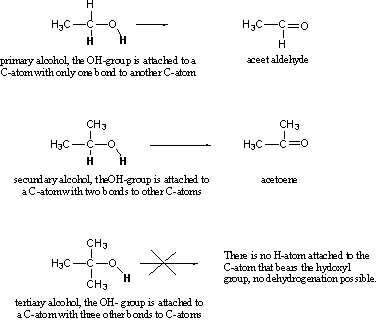
Scheme 3
A second general aspect of chemical reactions is the influence of steric hindrance. Steric hindrance means that groups within the molecule are obstructing the reaction that has to take place simply because these groups occupy space. This can prevent a reagent or a catalytic group of an enzyme, to reach the place where it has to react. It is a purely spatial effect but very realistic. When a reagent for instance like water can not reach the place where it has to perform its hydrolysis because large groups in the environment prevent its approach, then simply no hydrolysis will take place. In chemical terms: reactions can be inhibited or slowed down by steric hindrance.
The influence of steric hindrance again can be demonstrated nicely by the influence of the 17a-methyl group. For that reason the methyl groups at C13 and C17 have been drawn fully in the structural formulas at the left side of Scheme 1 and not just as CH3. When drawn with all their H-atoms it becomes clear that a methyl group needs space, at least more than just a H-atom. The steric hindrance of the two methyl groups at C13 and C17 makes it more difficult for reactive groups of the enzyme to approach the 17 hydroxyl group in the 17a-methyl anabolics. In 17a-H compounds approach from the bottom is still possible.
The increased steric hindrance in the 17a-methyl compound is also the reason why the 17 hydroxyl group is converted less easily into a glucuronate or sulphate. For this reason 17a-methyl anabolics are removed slower from the body and can exercise their effects longer.
It should be noticed that the reaction of the C17b- hydroxyl group to a glucuronate or a sulphate takes place at the O-atom and not at C17. Reactions at the O-atom are slowed down but not impossible.
The 17a-methyl group has a double function, it prevents for chemical reasons the conversion of the C17 hydroxyl group into a C17 carbonyl group and it slows down for steric reasons the conversion of the hydroxyl group into a glucuronate or sulphate. On the other hand, the steric effect of this methyl group is relatively small and it does not prevent a good interaction with the androgen receptor.
The interaction of the receptor with a 17a-methyl anabolic steroid probably will be slightly differ from that of the corresponding anabolic steroid with just a 17a-H-atom but in general all interactions will take place in a comparable way. An even better situation appears when changes in the anabolic steroid can be so that enzymatic transformations have become impossible or difficult while at the same time the interaction with the receptor has improved. This is rather difficult but not impossible. Examples can be found in anabolics with 17a-methyl or ethyl groups.
In Chapter 7 it has been shown that extra methyl groups can be beneficial for the binding with the receptor. The enlarged surface of the methyl containing molecule enables more Van der Waals forces for better interaction with the receptor and this is usually beneficial for the anabolic effect.
It is a well known fact that 17a-methyl anabolic steroids have an increased anabolic effect, but also strongly enhance the risk for liver damage. It is difficult to find good reasons for this liver damaging effect in the literature, but it is very realistic and warnings can be found everywhere. Speculations about the reason for this effect may be that first of all the 17a-methyl compounds are different compounds with their own properties. They circulate longer in the body and have more time to exercise their harmful effects. A different metabolism may also lead to other, maybe harmful, metabolites, which may be toxic for the liver.
|
|