Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 23/3/2007
10. Enzymatic Reactions of Anabolic Steroids
Aede de Groot, Willem Koert
It is a well known fact that anabolic steroids can undergo many enzymatic transformations in the body. These transformations are mostly undesired and many tricks have been invented to prevent them. Transformations in the stomach, intestinal tract and liver can be avoided by injecting the anabolic preparation, but this way of administration is not popular. It is easier to take an anabolic orally. Much research has been carried out to develop such anabolics.
In this chapter we first will discuss the relevant transformations of anabolics that can be carried out by enzymes. Later we will look upon these transformations a bit more closely and discuss the possibilities how to prevent them. We first will look at transformations which take place at the C-atoms of the steroid skeleton of the anabolic steroid. These are especially the reactions which take place at the carbonyl group of C3, with the hydroxyl group at C17 and with ring A of the steroid molecule.
After that also a number of enzymatic derivatization reactions will be mentioned. Derivatization reactions are reactions that take place with the substituents that are attached to the steroid skeleton. Especially transformations of the hydroxyl groups at C3 and C17 to sulfates, glucuronates and esters are important.
In Chapter 10 the role of enzymes in the metabolism of the sex hormones testosterone, dihydrotestosterone, estrone and estradiol will be mentioned. In the chapters 12 and 13 we will present a closer look at enzymatic transformations of anabolic steroids and we will see what has been done to prevent them.
Cytochrome P450 enzymes
The biosynthesis of the sex hormones starts with the oxidation of the side chain of cholesterol, which is catalyzed by the enzyme cytochroom P450scc. Oxidation is this case means the introduction of oxygen into the compound. Cytochrome P450 enzymes need a heme molecule as co-enzyme. The heme contains iron, which together with oxygen takes care of the oxidation.
Cytochrome P450 enzymes are very important for the metabolism and they occur everywhere in the body. They are especially abundant in the liver, where they oxidize a large variety of compounds. Cytochrome P450 enzymes introduce oxygen in carbon compounds by way of a hydroxyl group. This hydroxyl group then can bind with glucuronic acid or with sulfate, which make compounds much more polar so that they become soluble in water. In this way undesired compounds can be excreted from the body either via the kidneys with the urine or via the bile and the intestines.
Cytochrome P450 enzymes that work at other places than the liver are often much more specific and can oxidize only one, or a limited number of similar compounds at only one place in the molecule. This type of cytochrome P450 enzymes is usually specified by an additional code, which is related to their function. The indication scc in the cytochrome P450scc enzyme means cleavage of the cholestrerol side-chain and that is the only oxidation it can do. This cytochrome P450 enzyme oxidizes the side chain at C20 and C22 by the introduction of two hydroxyl groups. The same enzyme then breaks the chain between these two atoms under formation of pregnenolone (see Scheme 1).
The indication scc in the cholesterol oxidizing cytochroom P450scc mean cleavage of cholesterol side chain, and that is the only oxidation it can do. This cytochrome P450 enzyme oxidizes the side chain at C20 and C22 by the introduction of two hydroxyl groups. The same enzyme then breaks the chain between these two atoms under formation of pregnenolone (see Scheme 1).
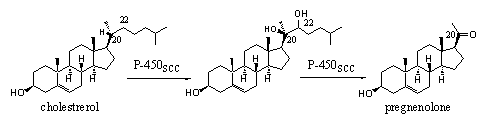
Scheme 1
The enzyme cytochrome P45017a catalyses the introduction of a hydroxyl group at C17 in pregnenolone on the aside of the molecule. This enzyme can catalyse the same reaction in the resembling steroid progesterone (see Scheme 2).
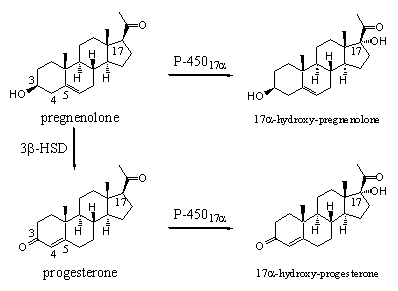
Scheme 2
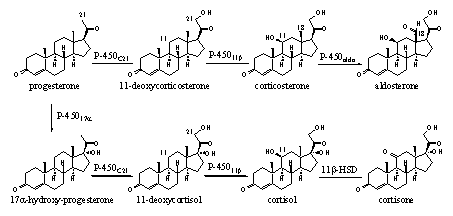
Several cytochrome P450 enzymes are involved in the biosynthesis of the cortico steroids, which play a role in the metabolism of sugars, proteins and fats and in the mineral household of the body. In Scheme 3 we see the introduction of a hydroxyl group at C21 in progesterone and in 17a-hydroxyprogesterone, catalyzed by cytochroom-P450C21. The introduction of a b-hydroxyl group at C11 in 11-deoxycorticosteron and 11-deoxycortisol is catalyzed by cytochroom-P45011b. Both cytochrome P450 enzymes accept both substrates also in this case.
Scheme 3
Click for larger pic
Cytochrome P450aldo catalyzes the oxidation of the C18 methyl group to a carbonyl group. Another name for this functional group is an is aldehyde, which is characteristic for aldosterone and is also used to characterize this enzyme. Furtheron we will encounter more cytochrome P450 enzymes that are involved in steroid metabolism. We now know what they generally can do.
Oxido-reductase enzymes
In Scheme 3 also a reaction is indicated which is catalyzed by an enzyme called 11b-HSD. The full name of this enzyme is 11b-hydroxy steroid dehydrogenase. This is an enzyme which catalyzes oxidations and reductions. For this reason it belongs to the large group of oxido-reductases.
The oxidations which are catalyzed by this type of enzymes follow a different course than those of the cytochrome P450 enzymes. The latter introduce an O-atom into the molecule as a hydroxyl group by oxidation of a C-H bond.
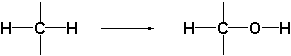
The oxido-reductases oxidize these hydroxyl groups further to carbonyl groups by removal of hydrogen. These enzymes are therefore also called dehydrogenases. To help them with this task, dehydrogenases use a different co-enzyme then the cytochrome P450 enzymes.
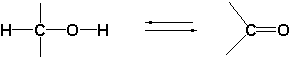
A second characteristic difference between reactions catalyzed by dehydrogenases and cytochrome P450 enzymes is, that oxidations catalysed by the latter only go in one direction while reactions catalysed by dehydrogenases can proceed in both directions. Cytochrome P450 enzymes introduce a hydroxyl group into a compound but they can not remove this group again. For this reason there is only a single arrow pointing to the right in the upper reaction equation.
Dehydrogenases oxidize the hydroxyl group to a carbonyl group but they also can reduce the carbonyl group again to a hydroxyl group. That is why there is a double arrow in the lower reaction equation, indicating that the reaction can proceed in two directions, it is an equilibrium reaction.
In the metabolism of anabolic steroids dehydrogenases are of utmost importance. They catalyze the oxidation of the hydroxyl groups at C3 and C17 to the corresponding carbonyl groups and reduce them back to the hydroxyl groups. The full name of 17b-HSD = 17b-hydroxy steroid dehydrogenase or 17b-hydroxy steroid oxido-reductase. The latter name is more appropriate for this enzyme because it indicates both capacities, to catalyse oxidation and reduction. The name dehydrogenase may lead to the conclusion the enzyme is only capable to abstract hydrogen from a compound. From the name it is not directly clear that it also can catalyse the reverse reaction and add hydrogen to the compound but it should be clear by now that this is indeed the case.
The 17b-HSD catalyses the reduction of the C17 carbonyl group to a C17-b-hydroxyl group, which means that the hydroxyl group is pointing upwards and that the hydrogen atom is delivered from the bottom side of the molecule. There also exists a 17a-HSD = 17a-hydroxy steroid dehydrogenase. It creates a C17-a-hydroxyl group by delivering the H-atom from the top side of the molecule. The 3b-HSD and 3a-HSD enzymes catalyze the same reactions, but then with the carbonyl group at C3 (see Scheme 4).
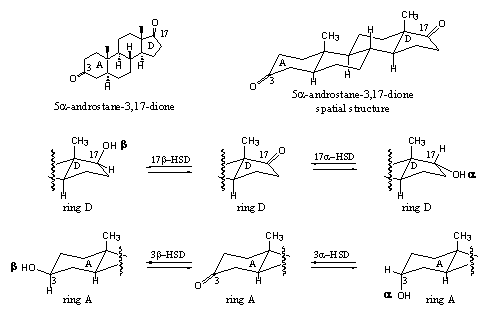
Scheme 4
Isomerase-enzymes
Compounds with the same bruto formula, which only differ from each other by the position of functional groups, are called isomers. Enzymes that catalyze the transformation of one isomer into the other are called isomerases. In the biosynthesis of testosterone there is one isomerase, called 3b-hydroxy steroid dehydrogenase / D5-D4-isomerase, important.
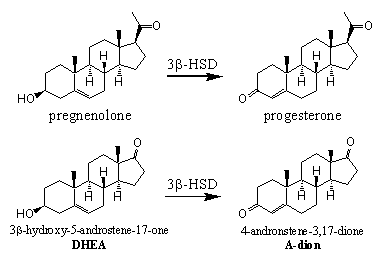
Scheme 5
This enzyme has a double task, it catalyses the oxidation of the C3 hydroxyl group to a C3 carbonyl group and the shift of the D5 double bond (the C5-C6 position) to the D4 position (the C4-C5 position). The enzyme can catalyze the oxidation and the shift of the double bond in several steroids, which contain a 3b-hydroxyl group and a D5 double bond.
First the oxidation takes place and next the isomerisation (see Scheme 5). This isomerisation is easier when there is a carbonyl group at C3 because structures with alternating single and double bonds, like O=C-C=C, are extra stabilised and therefore formed easier. The single arrow in the scheme indicates that this reaction is not an equilibrium and proceeds in only one direction.
Reductases
Structures with alternation single and double bonds, such as O=C-C=C, are called conjugated systems. These systems are more stable then those with the same functional groups further apart so that they can not influence each other. Usually conjugated systems are more stable then non conjugated ones, but in some reactions these systems are more reactive. One such a reaction is the reduction of a C=C-double bond which is conjugated with a C=O double bond. The reduction of this D4 double bond to a single bond is the reaction that converts testosterone like structures into dihydrotestosterone like structures. This is an important aspect in the metabolism of anabolic steroids.
The reduction of the D4 double bond can take place from two sides, just as in the reduction of carbonyl groups. The introduction of H-atoms at C5, can be catalysed by 5a- and 5b-reductases, giving 5a and 5b steroid skeletons respectively (see Scheme 6). The enzyme 5a-reductase or 5AR catalyzes the introduction of a H-atom in the 5a position in testosterone for the formation of dihydrotestosterone. Also this reaction proceeds in only one direction.
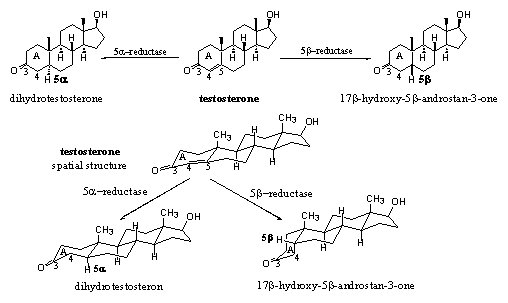
Scheme 6
The introduction of a H-atom in the 5b-position, catalysed by a 5b-reductase, creates another compound with a totally different shape of the left side of the molecule. This molecule is not biologically active.
Aromatase
The female sex hormones estrone and estradiol possess a peculiar aromatic ring A. This term, and the name aromatase for the enzyme which catalyses its formation, do have a far relation with aroma. Simple compounds with a similar aromatic structure as in ring A of estrone and estradiol do have a strong aroma. Representatives of aromatic compounds are benzene and toluene (see Scheme 7) and both compounds have a strong smell. Toluene is a solvent in the chemical industry and benzene is a notorious pollutant causing cancer.
In chemistry the term aromatic has got its own completely different meaning. Aromatics are compounds with alternating single and double bonds in a six membered ring. These compounds are remarkably stable, which makes their formation an easy process. The enzyme aromatase takes care of the formation of such an aromatic ring A in estrone and estradiol.
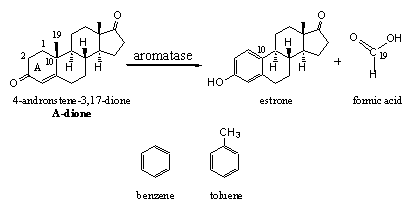
Scheme 7
The biosynthesis of estrone and estradiol takes place starting from A-dione or testosterone and it is clear that for aromatisation the C19 methyl group has to be removed. Carbon has only four bonds and in A-dione and testosterone all four bond are occupied. There is no place for an extra double bond at C10 because then C10 would need five bonds and C-atoms with five bonds do not exist. So one bond has to be removed first and the easiest is the one that binds the C19 methyl group.
The removal of this methyl group is carried out by a complex of cytochrome P450 enzymes with the code P450aromatase or aromatase. With the help of this enzyme the methyl group is first oxidized toa hydroxyl group and next to a carbonyl group, an aldehyde. Then this aldehyde is removed as formic acid together with a H-atom from C1 and aromatisation of ring A. In chapter 13 we will tell more about the mechanism of this reaction.
Prevention of aromatisation or better the prevention of the formation of estrogens is important for the treatment of estrogen dependent cancers. For that reason much research has been carried out to find aromatase blockers. Also for bodybuilders, aromatisation of anabolic steroids causes a tedious side effect. The resulting estrogens stimulate breast formation in man (gynecomastica). An aromatase blocker also may be of help in such cases.
The enzymatic reactions that have been mentioned above are all connected with transformations of C-atoms of the steroid skeleton. In the beginning of this chapter we have already mentioned that there are also a number of important enzymatic reactions which take place with the substituents. Especially the hydroxyl groups at C3 and C17 are involved in transformations to glucuronates and sulfates. Also the hydrolysis of esters of these hydroxyl groups are important in the biochemistry of anabolics.
Sulfatases
All kinds of carbon compounds have to be removed from the body sooner or later. Anabolic steroids contain many apolar structural elements like CH2- and CH3-groups and only a few polar OH- and C=O-groups. Steroids are predominantly apolar and little soluble in water. Also such apolar compounds have to be removed from the body and this generally can take place only after improvement of their polarity and solubility in water (read in the blood and urine). One way to increase the polarity of compounds is to attach polar groups. Mostly ionic groups or groups that contain many OH-groups are used for this purpose. In our body sulfate groups, derived from sulfuric acid, and glucuronic acid, derived from the sugar glucose, are applied for this purpose.
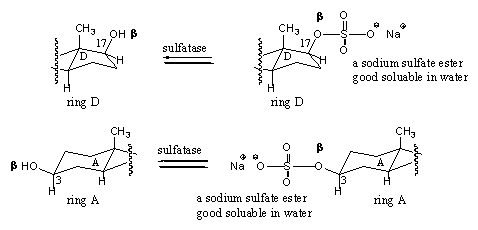
Scheme 8
A sulfuric acid derivative reacts in the body with one of the hydroxyl groups in the steroid and in this way it is converted into a sulfate ester. The reaction is catalyzed by sufatases. Especially the 3b OH-group and the 17b OH-group in steroids are converted into sulfates. These sulfates also can be hydrolysed again to the original hydroxyl groups, so these reactions are equilibria (see Scheme 8).
Glucuronidases
Glucuronides are also metabolic derivatives of steroids, which are biosynthesized to increase their polarity. Glucuronides are water soluble products, which can be excreted by the kidnies dissolved in the urine. Glucoronides are glycosides, which means that the steroid is bound to a sugar molecule with a glycosidic bond. A glycosidic bond is different from an ester bond and the two should not be confused. The general name of this type of bond is an acetal.
Acetals have not been described before in this book but this group is introduced here because also many anabolic steroids are marketed as acetals. A glycoside is an acetal of a sugar molecule. Glycosidic bonds (acetals) are common for carbohydrates.
The formation and hydrolysis of glycosidic bonds is catalyzed by glycosidases. The formation and hydrolysis of glycosides of glucuronic acid is catalysed by glucuronidases, a subclass which only catalyses the formation and hydrolysis of glucuronic acid derivatives. Also these reactions are equilibria. Glucuronic acid is obtained by oxidation of the CH2OH group (C6) of the sugar glucose to a carboxylic group (COOH group). The glucuronide is then obtained by reaction of the hydroxyl group at C1 of glucuronic acid with a hydroxyl group of the steroid (see Scheme 9).
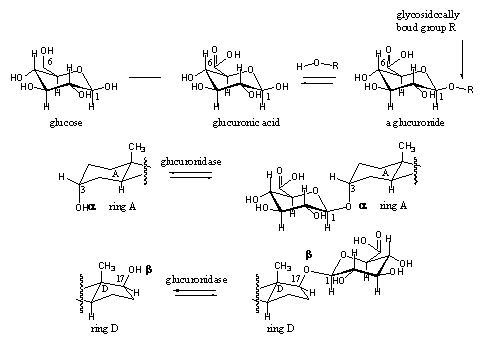
Scheme 9
The 3a-hydroxyl group in the steroid is transformed preferentially into a glucuronide. This in contrast to the 3b-hydroxyl group. which is preferentially transformed into a sulfate ester. Also the 173b-hydroxyl group is often derivatized to a glucuronide (see Scheme 10).
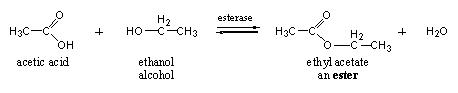
Scheme 10
Esterases
Esterases catalyze the formation and hydrolysis of esters. Esters are obtained by reaction of a carboxylic acid with an alcohol under formation of water. A simple example is the formation of ethyl acetate from acetic acid and ethanol (normal alcohol). The reverse reaction is also catalysed by esterases, namely the hydrolysis of esters with water to the carboxylic acid and the alcohol, the reaction is an equilibrium reaction (see Scheme 8).
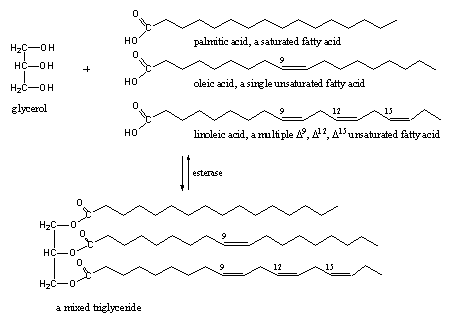
Scheme 11
Steroid hormones do not occur in the body as esters and enzymes also will not convert steroids into esters. Nevertheless we mention esters and esterases here because these enzymes can hydrolyze esters of anabolic steroids. Esters of anabolic steroids are often injected as drugs that are converted afterwards by esterases into the actual working anabolic.
Esters are found everywhere in our body and consequently there are many esterases to do something with these esters. All fats are esters of the tri-alcohol glycerol (a triol) and a large variety of fatty acids. Glycerol can be esterified three times with the same fatty acid, but also mixed triglycerides are common (see Scheme 11).These fatty acids may be saturated or may contain one or more double bonds. Multiple unsaturated fatty acids seem to be good for our health.
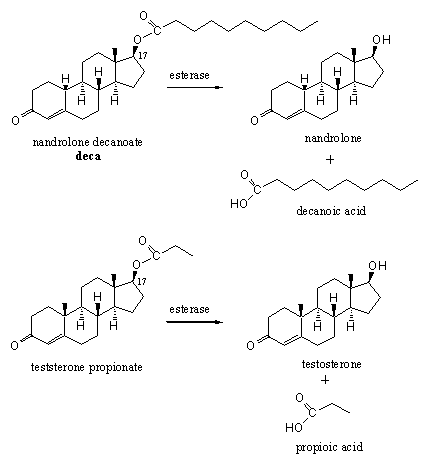
Scheme 12
Anabolic steroids can be deposited (injected) in (fat) tissue as an ester. Esterases slowly hydrolyze these esters and mobilize the real drug. Examples like deca and testosterone propionate are widely known but many variations have appeared on the market (see Scheme 11). There is also much opportunity for variation because in principle every hydroxyl group in the steroid can be esterified with any available carboxylic acid. For anabolic steroids especially injections with esters of the C17 hydroxyl group have proven to be effective. The right combination is determined by conditions like fat solubility, water solubility, transportation and vulnerability for metabolic transformations.
|
|