Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 7/10/2006
4. The structural Formulas of Testosterone
Aede de Groot, Willem Koert
In chapter 2 we have shown the binding possibilities and binding angles of C-, O- en H-atoms and it was demonstrated how to construct simple carbon compounds from single atoms. In the same way it is possible to build much bigger and more complicated molecules like testosterone from their constituting atoms. For testosterone the result is depicted in Figure 1.
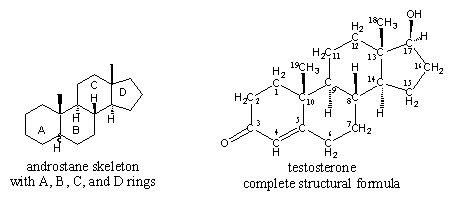
Figure 1
To construct a molecule like testosterone is not that easy and rules have to be followed to reach the correct result. Therefore the international rules for drawing steroids, and in particular of testosterone, will be treated first.
A closer look to the structural formula of testosterone learns that indeed all C-atoms have four bonds to other C-atoms, to H-atoms, or to O-atoms. Of the 19 C-atoms in testosterone, 17 C-atoms constitute the skeleton of the molecule. This characteristic steroid skeleton consists of four rings. Three six membered rings and one five membered ring, indicated with the capitals A, B, C and D, are fused together to a rigid framework. This framework is called an androstane skeleton and it is indicated also in Figure 1.
The C-atoms of the steroid skeleton are numbered as indicated in the complete structural formula of testosterone. Most of the C-C bonds are single bonds but in testosterone there is one C=C double bond between C4 and C5 and at C3 there is a C=O double bond.
The rings are coupled to each other in a so called trans way, which means that on all ring fusion points (C-atoms 10, 9, 8, 14 and 13) the fourth bond is alternatingly pointing upwards and downwards. This fourth bond at these points is not necessary to maintain the framework of the skeleton and in testosterone it is occupied by H-atoms or by methyl groups (CH3-group). The trans ring fusions make the steroid skeleton rather ridged and allows only minor movements at the ends of the system.
|
Figure 2 |
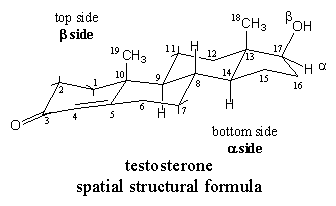
The one or two bonds of the C-atoms, which are not necessary for bonding the steroid skeleton itself, are sloping or pointing straight upwards to the top side, the b-side, or downwards to the bottom side, the a-side of the molecule. This can be noticed clearly in the spatial structural formula in Figure 2, from the short bonds at C1, C2, C7 and C11, from the bonds that connect the methyl groups at C10 and C13 and from the bond to the OH-group at C17.
The structural formula in figure 2 is called a spatial structural formula. Such formulas are useful to give an impression of the shape of the molecule. This spatial formula shows that the steroid skeleton is nearly planar and that the methyl groups at C10 and C13 are pointing upwards from the plane of the molecule. Also the flattened area around the double bond between C4 and C5 can be noticed is this formula.
|
Figure 3 |
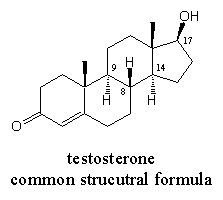
For a less confusing presentation of structural formulas, the numbering of the C-atoms is omitted completely or for the biggest part. Also the C- and H-atoms are not drawn when this is not necessary, as in the formula in Figure 3. It is assumed that there is a C-atom on each corner and at the end of each bond in the formula and that the remaining bonds on the C-atoms are occupied with H-atoms. Only deviating situations are indicated. In common structural formulas, the b-bonds are drawn with a thick or wedge-shaped line, the a-bonds are indicated with a hatched line as shown in Figures 1 and 3.
The H-atoms are generally only indicated in the structural formula when it is important to know their direction as at C9, C8 and C14. Also the H-atom at C17 is not drawn. When the OH group is drawn upwards and when no other groups are connected to C17, it is clear that the remaining bond is occupied with an H-atom that is pointing downwards.
|
|