The Anabolics Book
Willem Koert
Aede de Groot
Wageningen, 17/11/2008
21. The Real Anabolic Steroids
Aede de Groot, Willem Koert
Until this chapter all attention was focused on prohormones, hormone derivatives and designer steroids in food supplements. In this book we also have shown many real anabolic steroids, mostly as an example how to avoid metabolic transformations or detection. In this chapter we pay full attention to these real anabolic steroids.
The high-days of steroid chemistry lay approximately between 1950 and 1970. Attention in that period in the pharmaceutical industry went especially toward the development of steroids for treatment of hormonal diseases, hormone therapy, post menopause treatment, anti conception, anaemia, osteoperosis and prostate and breast cancer. These applications necessitated long lasting treatments with steroids and in such cases side effects should be absent. Application should be simple and preferably orally. Steroids should be effective during relatively long periods, so that application would not be necessary too often. This means that metabolic inactivation should not take place quickly. Also among bodybuilders such properties are highly appreciated, but of course they are primarily interested in the anabolic properties of steroids.
In the course of the years hundreds of steroids have been prepared by the pharmaceutical industry, in a continuous attempt to discover the ideal anabolic steroid. A few hundred of these steroids have been tested and/or patented. Many have reached the market, have disappeared again or have ended up in illegal circles.
From this large number of anabolic steroids we have chosen a selection, based on two considerations: Bodybuilders and weightlifters always have paid much attention to anabolic steroids. Several books about this subject have appeared, mostly mend for users. The anabolic steroids that are mentioned in the books of Duchaine [1] and Llewellyn [2] are discussed in this chapter. Also competing sportsmen and -women are highly interested in (undetectable) anabolic steroids for performance enhancement. The use of anabolic steroids is prohibited to prevent forging in sports competitions. The World Anti Doping Authority (WADA) has published a list of forbidden anabolic steroids [3] and these steroids are discussed as well.
The subtle difference between steroids in food supplements and real anabolic steroids has been mentioned already several times in Chapter 19. Many prohormones and steroids in food supplements are in fact straightforward anabolic steroids. The WADA is not fooled so easily as authorities or users and this organization has placed many of them on its list of forbidden steroids. This interweaving of steroids in food supplements and real anabolic steroids is visualized in this chapter.
This has been achieved with a code under the structural formula of all steroids that are for sale in food supplements and that are also mentioned in the list of the WADA. To mark the steroids in food supplements we have used the number they have got in Chapter 19. The names of the steroids that occur in the list of the WADA are printed in red. It appears that among the 80 real anabolics that are discussed in this chapter, 20 steroids are for sale in food supplements and are also mentioned in the list of the WADA. We have counted only the basic steroid molecules (for instance nandrolone), all the derivatives (for instance nandrolone esters) are not included in this number.
The 80 anabolic steroids in this chapter are divided in smaller groups, to make the discussion easier. This subdivision has been organized in a similar way as in Chapter 19. Large groups have been subdivided based on their chemical structure. In this way we have composed 11 groups, each with a well known anabolic steroid as its icon.
1. Testosterone-type steroids
2. 19-Nor androstenes
3. 17a-methyl androstenes
4. 19-Nor-17a-methyl- en -ethyl-androstenes
5. Dihydrotestosterone-type steroids
6 D1-Testosterone-type steroids
7. Androstenes with more then one double bond
8. 19-Nor and 17a-methyl analogs of steroids with more then one double bond
9. Steroids with an extra hydroxyl group at C4
10. Chloro containing steroids
11. Steroids with a heterocyclic ring at ring A
Group 1. Testosterone-type steroids
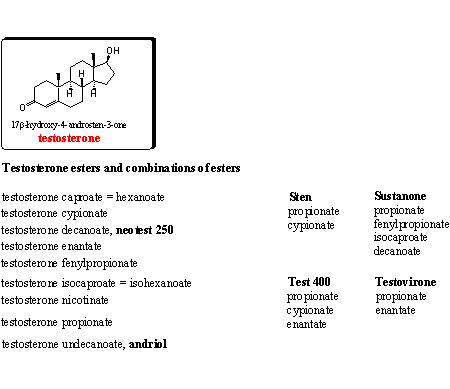
Testosterone and many of its esters are on the market for a long time as anabolic preparations. In Chapter 16 we have already paid attention to the testosterone esters. Testosterone itself is metabolized quickly in the body and therefore users prefer these esters. After injection a slow release to the blood takes place, where esterases hydrolyze the ester. In this way a higher testosterone level is ensured for a longer period.
The body itself produces the so called endogenous anabolic steroids. These are the regular intermediates and end products of the biosynthesis of testosterone (see Chapter 11).
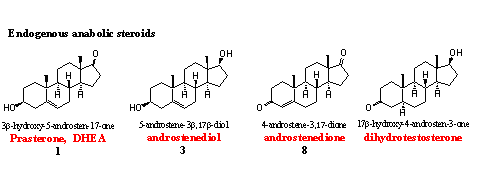
When doping hunters discover deviations from the normal concentrations of these steroids in competing athletes, the WADA considers this as a positive doping test. This is also the case when the doping hunter can prove that the steroid is administered. The WADA indicates such a steroid as being from exogenous origin. The same two rules apply for all the metabolites of the endogenous anabolic steroids mentioned below.
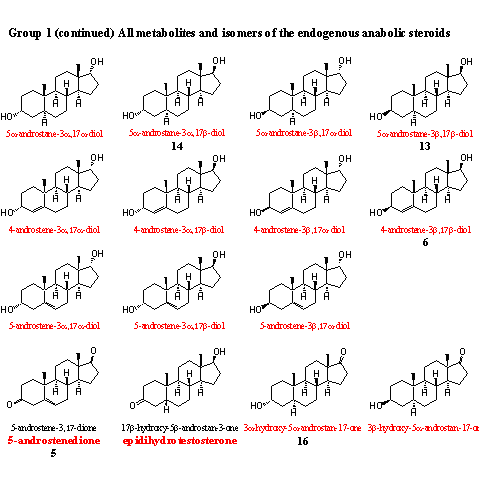
In this group of intermediates and metabolites already eight steroids are present that are sold also in food supplements. It is not important whether these steroids have any physiological effect from themselves, or whether they are transformed in the body into a real anabolic steroid. The WADA considers all deviations from normal concentrations of these steroids as suspect. Competing athletes that are controlled regularly for doping abuse should not use these prohormones.
Group 2. 19-Nor-androstenes
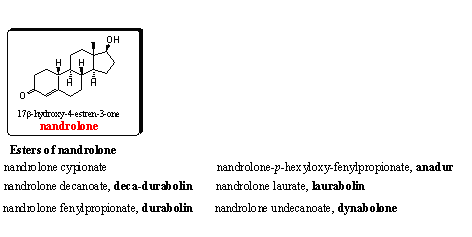
Nandrolone and many of its esters are popular steroids with good anabolic properties. For nandrolone itself this is already known from 1930. The nandrolone esters have, just as the testosterone esters, a longer lasting activity. [4] Also the nandrolone esters are discussed in Chapter 16.
In principle nandrolone can aromatize, but this happens only under special circumstances and in small amounts. The normal aromatization of androstanes with the enzyme aromatase can not take place in nandrolone because the 19-methyl group is absent. Other enzymes have to oxidize ring A (remove two hydrogen atoms at C1 and C10) and then, after enolization of the C3-carbonyl group, the aromatic ring A is obtained.
Reduction of the D4 double bond by the enzyme 5AR can take place in a similar way as in testosterone. The androgenic activity of dihydronandrolone and its analogs is lower then that of the corresponding testosterone steroids. This means that less side effects occur in tissues where 5AR is active and converts nandrolone in dihydronandrolone. This results in a better ratio between anabolic and androgenic effects for nandrolone, in comparison with testosterone.
The four nandrolone analogs and metabolites in the figure are on the WADA list. Steroids 8 and 9 are also on the market as prohormone for nandrolone and have been discussed Chapter 19.
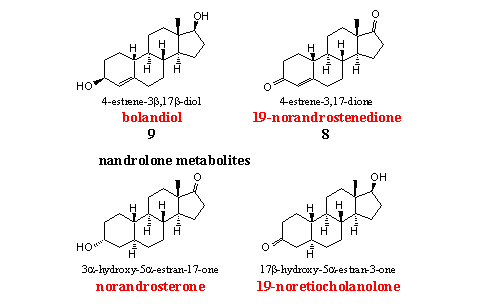
Doping hunters use norandrosterone and 19-nor etiocholanone, the two main metabolites of nandrolone, to detect abuse of this anabolic. We have already mentioned the fact that small amounts of nandrolone may occur naturally in the body as a by product of the aromatization of testosterone and androstenedione. The limits of the IOC for the allowed concentrations are still a matter of discussion. [5]
Group 3. 17a-Methyl-androstenes
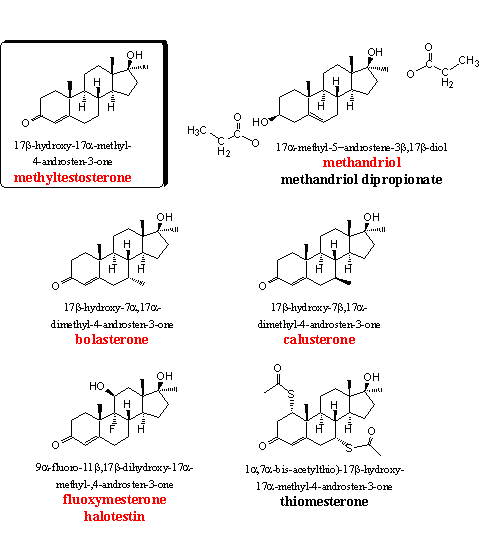
The steroids in group 3 all contain the well known 17a-methyl group. This group inhibits the oxidation of the 17b-hydroxyl group and the larger steric hindrance also prevents an easy oxidation of C16. Also the reaction of the 17b-hydroxyl group with glucuronic acid or sulfate suffers from steric hindrance. For these reasons the 17a-methyl anabolics are removed less quickly from the body and can be taken orally.
Methyltestosterone is the basic steroid in this group. We have not found this steroid in food supplements, but a prohormone for methyltestosterone (methyl-4AD, Chapter 19, steroid 12) is for sale in food supplements. Methyltestosterone is a normal anabolic steroid, and is one of the first 17a-methyl steroids that appeared on the market. Researchers use this steroid, together with testosterone propionate, as a standard for comparison of the anabolic and/or androgenic activities of other steroids.
Ring A in methyltestosterone has the same structure as in testosterone, and therefore the same metabolic transformations can take place. This means that methyltestosterone can aromatize to give methylestradiol. The side effects of methylestradiol are fat and water retainement and gynaecomastia, and it is more active in these effects then estradiol itself.
The enzyme 5a-reductase can reduce methyltestosterone to methyldihydrotestosterone (mestanolone, see group 5), which is responsible for the androgenic side effects.
The enhanced liver toxicity of 17a-methyl steroids has been mentioned already several times and, users should not underestimate this danger of methyltestosterone. [6] [7]
The anabolic steroid methandriol shows reasonable anabolic properties. It does not aromatize quickly and also reduction by 5AR is not directly involved. The reason for this is that a 3b-HSD enzyme first has to oxidize the hydroxyl group at C3 to a carbonyl group and has to shift the double bond to the conjugated D4 position, in a similar way as in DHEA. Only after these transformations metabolic reduction or aromatization becomes possible. For that reason methandriol does not show many side effects. When these two conversions take place, methandriol is converted into methyltestosterone and the latter is indeed one of the metabolites of methandriol. The side effects of methyltestosterone then become apparent, although to a lesser extent. The 3-propionate and the 3,17-dipropionate esters of methandriol are also for sale. Tests show a reasonable activity for the 3-propionate. [8]
In earlier times several studies have been published in which the anabolic and androgenic activities of popular anabolic steroids are compared. The results of a few of these studies are shown in tables, with rats as test animal. [9] They give an indication about the relative activities of anabolic steroids in human, but the results should be considered with reserve.
Ratio anabolic : androgenic |
|
17a-methyl-4-chloortestosterone (clostebol) |
5.4 : 2.7 |
17a-methyl-5-androstene-3,17-diol (methandriol) |
1.1 : 0.7 |
17a-methyl-19-nortestosterone (methylnortestosterone) |
4.2 : 5.3 |
1-methylandros-1-enolone-acetate (primobolan) |
0.004 : 4.8 |
This study confirms the relatively modest anabolic activity of methandriol, which had already been found in earlier studies. The same is true for the ratio between the anabolic and androgenic activity, which is around one. [10] From tests on human is concluded that methandriol has relatively weak anabolic properties and that it has no clinical advantages above testosterone. [11]
In a British patent from 1960, the good anabolic properties of 7-methyl androstenes are mentioned for the first time. [12] The extra 7-methyl group in bolasterone and calusterone enlarges the surface of the molecule so that a better interaction with the androgen receptor becomes possible. The 7a-methyl group points downward and protects the D4 double bond for reduction by the enzyme 5AR. Reactive groups in the active cavity of the enzyme are severely hindered in their approach from below to the double bond, and reduction to a 7a-methyl dihydrotestosterone analog does not take place. The 7b-methyl group is placed horizontally, in the plane of the steroid skeleton, and hinders this reduction much less.
The anabolic and androgenic properties of bolasterone have been investigated extensively in the sixties and they have been compared with other steroids. Some of the outcomes are collected in Table 2. [13]
Ratio anabolic : androgenic |
|
7a,17a-dimethyltestosterone (bolasterone) |
4.2 : 1.3 |
7a,17a-methyl-4-hydroxytestosterone (oxymesterone) |
1.8 : 0.36 |
7a,17a-methyl-androstanolone (mestanolone) |
0.8 : 1.0 |
9a-fluoro-11b-hydroxy-17a- |
3.8 : 1.4 |
In 1964 Upjohn claims bolasterone to be a much more powerful orally available anabolic steroid then methandrostenolone and oxymetholone or any other known steroid [14], with a favourable ratio between anabolic and androgenic activity in the rat. [15]
Fluoxymesterone or halotestin is also a familiar anabolic steroid. The good oral activity of 11b-hydroxy-, 11b-hydroxy-9a-fluoro- and 11-keto-9a-fluoro-steroids was discovered already in 1956. [16] In a later publication the anabolic activity of fluoxymesterone was mentioned comparable to that of methyltestosterone and testosterone propionate. [17] User manuals are less positive about fluoxymesterone and mention its high androgenic activity, it is usually not recommended.
Thiomesteron (protabol) is characterized by Duchaine as an elusive steroid that he never has been able to find. [1] It has been synthesized and tested for the first time by Merck in 1964 and they describe the steroid as having excellent anabolic activity with few side effects. [18] [19]
In Table 3 several activities of a number of well known anabolic steroids are compared in the rat as test animal. [20] The minimal effective doses, which cause a significant effect, are shown. The smaller this dose the more active the steroid. The activities that have been tested are:
a. Weight loss of the testes, which tests the influence on the HPT axis (see
Chapter 20)
b. de Weight gain of the prostate, which is also a measure of the androgenic activity
c. Weight loss of the seminal vessels, which is a measure of the androgenic activity
d. Weight gain of the levator anni, which is a measure of the anabolic activity
Testosterone shows all these effects at the lowest effective dose. The best result for a steroid is reached when the anabolic effects become apparent at a low dose, while at the same time the other three effects, which are responsible for the undesirable androgenic effects, appear at a higher dose. Nandrolone fenylpropionate, norboletone, bolasterone, norethandrolone, oxymesterone and metenolone acetate all give reasonable results for these effects.
a. |
b. |
c. |
d. |
|
oxymetholone |
100 |
400 |
1000 |
100 |
oxandrolone |
100 |
400 |
1000 |
200 |
methandrostanolone |
100 |
1000 |
1000 |
200 |
nandrolone-fenylpropionate |
< 40 |
100 |
200 |
< 40 |
norboletone |
< 40 |
100 |
200 |
< 40 |
ethylestrenol |
400 |
1000 |
100 |
|
ethylestrenol |
< 40 |
100 |
100 |
< 40 |
norethandrolone |
< 40 |
200 |
400 |
< 40 |
norethandrolone |
< 40 |
200 |
400 |
< 40 |
oxymesterone |
< 40 |
200 |
400 |
< 40 |
metenolone-acetate |
< 40 |
100 |
200 |
< 40 |
chlorotestosterone-acetate |
100 |
400 |
2000 |
100 |
testosterone |
< 40 |
< 40 |
< 40 |
< 40 |
stanozolol |
< 40 |
200 |
400 |
< 40 |
Group 4. 19-Nor-17a-methyl- and 17a-ethyl-androstenes
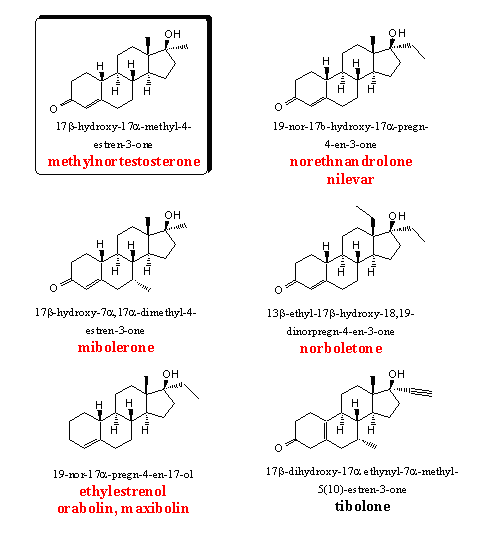
The 19-nor steroids show good anabolic and moderate androgenic activities and the 17a-methyl steroids possess a longer lasting oral activity. It was therefore obvious to synthesize and test steroids with a combination of these two structural elements. Not only methyl groups but also the larger ethyl and propyl groups have been introduced at C17. Tests have revealed that the highest activities are obtained with methyl and ethyl groups. With longer alkyl groups the activity quickly drops to lower levels [21] [22] and such steroids have not appeared on the market.
Also the methyl group at C13 has been replaced by longer alkyl chains and also here the methyl and ethyl groups give the best results. Nilevar and norboletone are the best known steroids of this type. In mibolerone an additional 7a-methyl group is present which prevents metabolic reduction of the D4 double bond.
Methylnortestosterone and norethandrolone are two early examples from this group of nor androstenes. Both 17a-alkyl substituted nor androstenes have been tested often together and norethandrolone usually is the most active anabolic, with also the best ratio between anabolic and androgenic activities. (see also Tables 1 and 3). [23-25]
Norboletone has been included regularly in these studies [26] (see Table 3) and its high anabolic and low androgenic activity is mentioned with appreciation. Later it was shown that the androgenic side effects of this designer steroid may be rather strong. Norboletone has been described already in Chapter 18
Mibolerone has an extra 7a-methyl group. Several 7a,17a-dimethyl-19-nor-testosterones have been synthesized and tested by Upjohn in the beginning of the sixties. [27] [28] The activities of mibolerone have been compared with those of fluoxymesterone in rats and apes as test animals. In a paper in Steroids is concluded that the anabolic activity of mibolerone is 14 times higher then that of fluoxymesterone in apes. In another paper they find an androgenic activity 14 times higher and an anabolic activity 41 times higher then that of methyltestosterone, when tested in rats. In the same paper is mentioned that the anabolic activity of mibolerone is 144 times higher then that of fluoxymesterone.
It has already been mentioned that researchers were surprised by the good anabolic activity of steroids without a C3 carbonyl group. In the androstane series desoxymethyltestosterone (DMT, see group 5 and Chapter 18) is a good example but also in the group of the 19-nor androstanes such desoxy steroids have been synthesized and tested, especially by Organon. [29]
Ethylestrenol is merchandized under the trade names orabolin and maxibolin. It is an orally available mild anabolic steroid. From the structure it immediately becomes clear that ethylestranol can not aromatize (no C3-carbonyl group and no C19-methyl group). Also 5a-reduction will not take place. That requires a double bond in conjugation with the C3-carbonyl group and the latter is not present here. Ethylestranol shows few side effects.
Tibolone has been investigated and patented also by Organon in the mid sixties. [30] Tibolone and its metabolites show a broad spectrum of activities as anabolic, estrogenic and progestagenic steroid. It is the active ingredient in Organons Livial, a preparation for post menopause treatment. It is also a good intermediate for the preparation of several other active steroids. Recently several patents for the synthesis of tibolone have appeared in the literature.
Group 5. Dihydrotestosterone-type steroids
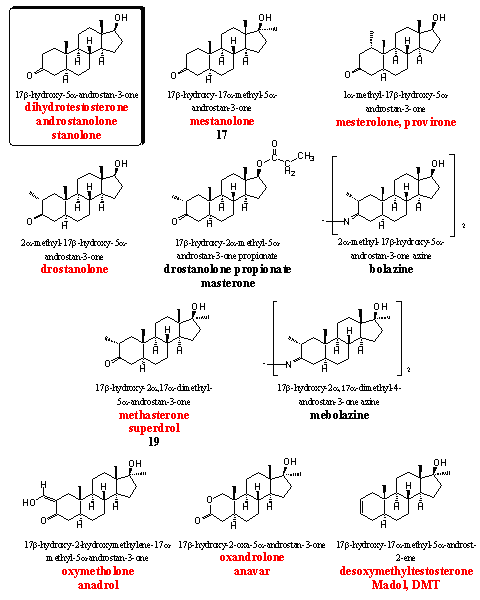
The steroids in group 5 all have a 5a H-atom, which means that these steroids do not aromatize. They all miss the D4 double bond, which is necessary for aromatization (see Chapter 14).
On the other hand, many of the steroids in this group will show strong androgenic effects. It is however difficult to predict all the activities of the steroids in this group. Tests and experiences of users are necessary to establish the ratio between anabolic and androgenic activities. Mestanolone, mesterolone and drostanolone all show androgenic properties in the first place.
The azines bolazine and mebolazine have been developed and patented in the beginning of the sixties in Italy. [31] Mexican researchers have established a three times higher anabolic activity for mebolazine in comparison with testosterone propionate. [32] Also the Italian researchers have found an anabolic activity for these azines that is higher than that of methyltestosterone. [33] [34] We have the impression that these derivatives are not for sale anymore.
Bolazine and mebolazine are not mentioned in the list of the WADA, but nevertheless they should not be used by active sportsmen. These derivatives will hydrolyze partly or completely to drostanolone and methasterone (better known as superdrol) under the acidic circumstances in the stomach and the latter two anabolics are in the WADA list. Users of bolazine and mebolazine for certain will show a positive doping test. The same counts for drostanolone propionate (masterone), esterases will set free drostanolone in the body as the active steroid, which will be traced down easily by doping hunters.
The synthesis of drostanolone and superdrol has been published already in 1956. [35] Shortly afterwards the synthesis of drostanolone, drostanolone 17-propionate (masterone), superdrol and oxymetholone was published by Syntex. [36] In this publication the strong oral anabolic and the relatively low androgenic activity of these steroids is mentioned. Also in later patents of Upjohn [37] and in the Italian patent [31] their high anabolic activity is claimed. However masterone has become a bit less popular lately and in the meantime superdrol has become notorious because of its liver toxicity. [38]
The best known anabolics in this group are oxymetholone, oxandrolone and madol (DMT). They all have a 5a-H-atom but they are not very androgenic and show good or very good anabolic activities.
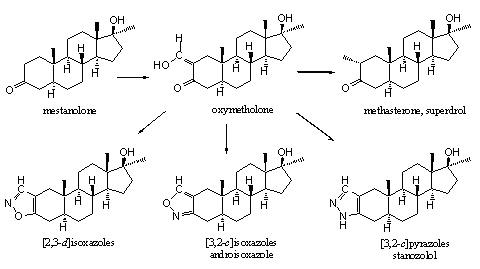
It is chemically not difficult to introduce a hydroxymethylene group in a molecule next to a carbonyl group. Also in anabolic steroids this has been carried out at C2, next to the carbonyl group at C3. In this way mestanolone is transformed into oxymetholone. [36] The obtained hydroxymethylene steroid then can be converted easily in other ring A substituted steroids. It is not surprising that several of such steroids have been synthesized, tested and merchandized. In Chapter 18 we have already shown the possibilities of oxymetholone as intermediate for the synthesis of many other designer steroids (see Chapter 18) and one of these schemes is shown here again (see Scheme 1).
Oxymetholone is a powerful anabolic steroid with relatively mild androgenic activities. It has been applied for a long time as a medicine for anaemia, but later epo became available as a better alternative with less side effects. Oxymetholone is still on the market as a medicine for muscle dystrophy in HIV patients.
Some serious side effects of oxymetholone are water retention and gynaecomastia, the latter in spite of the absence of the D4 double bond. For this reason the enzyme aromatase can not aromatize oxymetholone in a normal way. Arnold and Llewellyn [39] speculate that oxymetholone itself can interact with the estrogen receptor, because of the presence of a phenolic type hydroxyl group in ring A. In Scheme 2 a possible chemical explanation for this speculation is presented.
The hydroxyl group in methylestradiol is connected to an aromatic ring. Chemists characterize such a hydroxyl group as a phenolic hydroxyl group. The H-atom of this hydroxyl group is bound relatively weak to the O-atom. This means that it is relatively acidic (it will loose H+ relatively easily) and it is a good hydrogen bridge donor. A similar reasoning counts for the H-atom on the hydroxyl group in hydroxymethylene-carbonyl compounds. This hydroxyl group is even more acidic than a phenolic hydroxyl group. In addition there is the possibility of tautomerism in hydroxymethylene-carbonyl compounds. This means that in an equilibrium this H-atom can shift to the other O-atom with concomitant shifting of the double bonds. The situation in the right tautomer resembles that in methylestradiol rather closely. This may explain why oxymetholone itself can interact with the estrogen receptor. This is however speculation and we have not found literature to confirm this explanation.
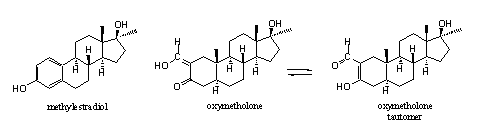
To prevent gynaecomastia by oxymetholone it is useless to inhibit the enzyme aromatase. This aromatization does not take place and probably is not necessary for bonding of oxymetholone to the estrogen receptor. To prevent gyneacomasia by oxymetholone it is necessary to block the estrogen receptor itself.
Oxandrolone (anavar) has been developed in the beginning of the sixties by Searle. [40] [41] Oxandrolone has an unusual ring A in its steroid skeleton, C2 is replaced by an O-atom and this converts at the same time the carbonyl group at C3 into an ester. This ester function is part of ring A and such a cyclic ester is called a lactone. This lactone still has a carbonyl group as a hydrogen bond acceptor for bonding to the receptor. A lactone is however not reduced like a carbonyl group and metabolic conversion at C3 (reduction to a hydroxyl group) does not take place.
Ring A of oxandrolone also can not aromatize. The O-atom has only two bonds and these are necessary to form the ring. There is no place for an extra double bond to form the aromate. Besides, also the D4 double bond is absent, which is a second reason why aromatization does not occur.
The 17b-methyl group inhibits oxidation of the 17a-hydroxyl group and hinders also bonding with glucuronic acid or sulfate. All together it is clear that oxadrolone is not metabolized easily. The anabolic is orally active and leaves the body unchanged after some time. [42]
The anabolic activity of oxadrolone is about six times that of methyltestosterone [43] [44], and it does not show many androgenic side effects. Oxandrolone in low doses is also suitable for women, without much risk for masculinization. The steroid does have a 17a-methyl group but it is not very toxic for the liver. In the USA it is approved by the Food and Drug Administration (FDA) for HIV patients.
Oxandrolone stimulates muscle development in young men. [45] They speculate that oxandrolone causes an increase of the androgen receptor concentration in muscles. The anabolic effect of oxandrolone has been shown to have a favorable effect also in elder men. [46] It stimulates muscle growth and reduces fat tissue. When the use of oxandrolone is stopped, the effect on muscles disappears within 12 weeks but the reduction of fat remains.
The designer steroid madol (DMT) has been discussed extensively in Chapter 18. The SARM like properties (see Chapter 20) of this steroid have been established recently. Madol was found also in food supplements [47], although this was not indicated clearly (See Chapter 19).
Group 6. D1-Testosterone-type steroids
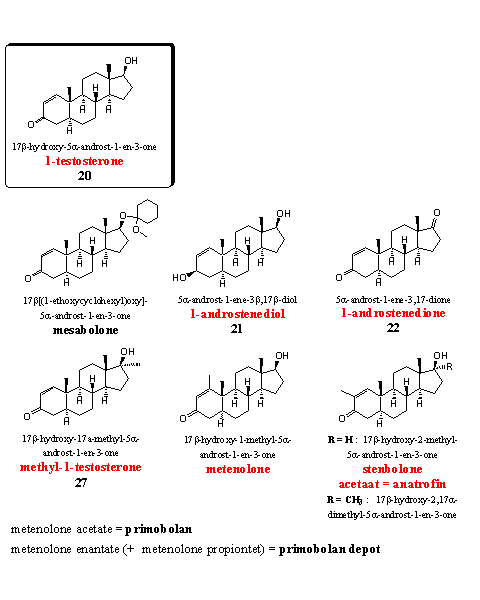
1-Testosterone (steroid 20) is a powerful anabolic itself, but producers advertise it as a prohormone. Mesabolone is an acetal of 1-testosterone (see Chapter 17). The acetal probably will hydrolyze to 1-testosterone in the stomach. The other two steroids on the upper row are prohormones for 1-testosterone, they are mentioned in Chapter 19 as steroids 21 and 22.
Also methyl-1-testosterone is mentioned in Chapter 19 as steroid 27. It is a strong anabolic but also for sale as a food supplement.
The steroids metenolone and stenbolone have an extra methyl group at the D1 double bond. The acetate (primobolan) and the ennantate (primobolan depot) are well known derivatives of metenolone. Both were developed and patented in the sixties by Schering. [48] [49] Metenolone and primobolan belong to the small group of orally active anabolics without a 17a-methyl group. They are therefore not very toxic for the liver. A drawback is the relatively high dose that is necessary, because metabolic transformations do occur, but slower then in testosterone. The longer active enantate is injected. Metenolone is a mild anabolic steroid with few androgenic side effects, it is popular with women.
Also stenbolone is introduced by Schering and later by Syntex in the beginning of the sixties. [50] [51] The acetate of stenbolone became known under the trade name anatrofin. [51] It is a mild anabolic that does not aromatize and it shows few androgenic side effects. A 17a-methyl variant is also known. [50] [52] This is a more powerful anabolic with only few androgenic side effects, but probably with a higher liver toxicity.
Group 7. Androstanes with more then one double bond
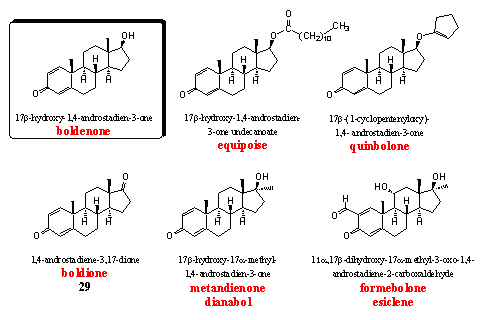
The steroids in this group have an extra D1 double bond in ring A, in comparison with testosterone. The best known steroids in this group are boldenone and especially its 17a-methyl analog dianabol. The introduction of the characteristic D1,4 dienone system in ring A was one of the first successful modifications of testosterone to improve its performance.
The androgenic side effects of these steroids are modest, at least at moderate doses. Aromatization does occur in D1,4 dienones. A possible explanation for this phenomenon has been mentioned in Chapter 14 (see Chapter 14). The enzyme aromatase probably is involved only in the first two oxidation steps, the formation of the C19 hydroxyl and carbonyl intermediates in the aromatization process. Thanks to the presence of the extra D1 double bond, these intermediates can loose easily formaldehyde or formic acid, under the influence of hydroxide (OH_). In this way the formation of aromatic ring A can take place without the help of aromatase. This is a speculative explanation but from a chemical point of view it is possible (see Scheme 2).
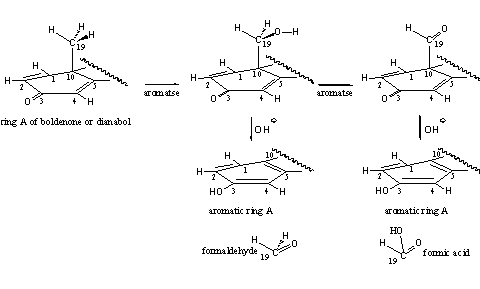
Boldenone itself and two of its derivatives, equipoise and quinbolone, are well known anabolics. [52] Equipoise is the injectable undecanoate ester of boldenone. Its activity resembles that of decadurabolin, the comparable decanoate ester of nandrolone. Eguipoise is a mild anabolic that originally has been developed for veterinairy use. The C17 hydroxyl group is esterified with an undecanoate fatty acid, which makes this derivative very apolar. After injection it is deposited well in fat tissues, that are also apolar. It stays long in the body in this way, just like deca, and doping hunters can find it during a long time after abuse.
Quinbolone is an enol ether derivative of boldenone, the enol ether structural element is located in the five membered ring that is connected to the C17 hydroxyl group. Enol ethers are not stable in acidic environments and boldenone will be set free already in the stomach after partly or complete hydrolysis of this enol ether. Quinbolone is the active principle in Anabolicum Vister, an orally active preparation, that is known especially in Italy. [53]
Boldione is patented as a prohormone for boldenone. A 17b-dehydrogenase has to reduce the C17 carbonyl group to a 17b-hydroxyl group in the body to obtain boldenone. [54] Equipoise, quinbolone and boldione are all precursors for boldenone. It has been shown that users of these three steroids excrete the same metabolites as after the use of boldenone itself. This is a prove for the fact that boldenone is the intermediate and the active steroid in all these steroids. [55] Boldenone is a mild anabolic with few androgenic side effects. Aromatization becomes only a problem at high doses.
Dianabol is the 17a-methyl analog of boldenone. It has been developed by CIBA [18] [25] [52] and it is one of the anabolic steroids with the longest users history. Weightlifters used it already in the mid fifties and in the sixties it also became popular in other sports. [56-57] Dianabol is a powerful orally active anabolic steroid with few androgenic side effects. Aromatization to 17a-methyl estradiol does occur (see above). Because methyl estradiol is more active than estradiol itself, this side effect of dianabol is also more tedious than the same side effect in boldenone and its derivatives. The 17a-methyl group also enhances the liver toxicity.
Formebolone is a bit a strange steroid. It induces local inflammations in the injected muscle, which as a consequence gets swollen and becomes bigger. This effect is the best visible in smaller muscles and lasts about five days. Bodybuilders use this effect to improve their appearance in competitions. [2] The steroid is mildly anabolic and weakly androgenic, it has been patented by Italians in the sixties. [58]
Group 8. The 19-nor and the 17a-methyl and ethyl analogs of steroids with more than one double bond
The steroids in this group all have no C19 methyl group and this has seduced people to compare these steroids with other 19-nor androstanes like nandrolone. This comparison does not hold water for several reasons.
All steroids in this group have a double bond between C9 and C11 and there is not such a double bond in nandrolone. This double bond makes the steroid flatter and more flexible, which enables a better interaction with the androgen receptor. For that reason the steroids in this group are more active.
The D9,11 double bond also inhibits aromatization and gynecomastia is not a side effect in these steroids. Nandrolone in principle can aromatize, although this effect is not very strong.
The conjugated system of double bonds (the structural element with alternating double and single bonds between C4=C5-C9=C10-C11=C12) stabilizes the D4 double bond. For this reason, the enzyme 5a-reductase can not reduce this D4 double bond and thus modify the activity of these steroids in sensitive tissues. Steroids in this group therefore usually show substantial androgenic side effects. 5a-Reductase will convert nandrolone into the less active dihydro nandrolone.
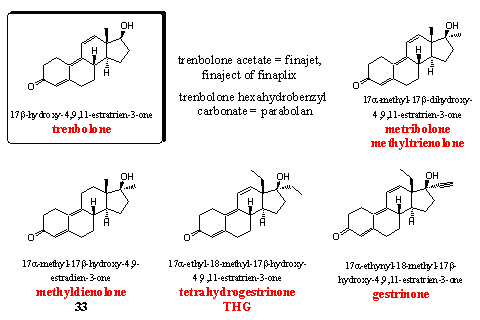
Trenbolone, the key steroid in this group, has been developed by Roussel-Uclaf [59] in the end of the sixties. Originally it is a veterinary preparation but it is also popular with bodybuilders. It is a strong anabolic steroid. We have already explained that trenbolone does not aromatize and that is an advantage over other strong anabolics like dianabol or testosterone. Trenbolone does have substantial androgenic side effects. These effects also cause hardening and definition of the muscles without water retaining. Trenbolone is available as an injectable acetate, and in former times also as a hexahydrobenzyl carbonate (Parabolan). Esterases convert these ester derivatives in the body in the active anabolic trenbolone.
Metribolone (methyltrienolone or methyltrenbolone) is mentioned also in the same patent of Roussel-Uclaf. [59] It is the most powerful anabolic steroid that is known today. Its activity is so high that it becomes difficult to compare it with, for instance, testosterone propionate or methyl testosterone. An activity of 30 000 times of that of methyltestosterone in mentioned in the literature. Methyltrenbolone is known in the scientific world under its codename R1881, and it is used often as comparison for the activity of other steroids. Methyltrenbolone is also the most toxic steroid for the liver that is known today. It is a dangerous steroid that is absolutely unsuitable for use by human. You will get problems for sure.
Methyldienolone is also a strong anabolic with relatively few androgenic side effects. Its activity is much lower than that of metribolone, but higher than that of methyl testosterone. Methyldienolon was also found in food supplements and we have discussed it already in Chapter 19 as steroid 33. [60] [61]
It is also not necessary to discuss THG again. This steroid is one of the main subjects in Chapter 18
Gestrinone has been developed originally as an oral anti conceptive and has been patented in the mid sixties for that purpose by Roussel-Uclaf. [62] Gestrinone has a relatively broad spectrum of activities. It is applied for treatment of endometriose. It deminishes spermatogenisis and it also has mild anabolic properties. For that reason it is mentioned on the list of the WADA. Gestrinone has been used by Pat Arnold as a starting material for the preparation of THG (see Chapter 18, Scheme 2).
Group 9. Steroids with an extra hydroxyl group at C4.
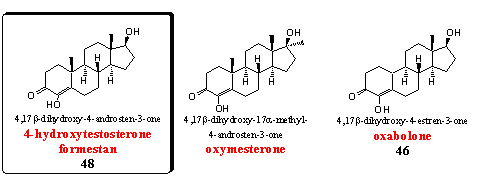
Steroids with an extra hydroxyl group at C4 are discussed also in Group 5 of Chapter 19. Some representatives in this group are aromatase inhibitors others have anabolic properties.
Formestan is the basic steroid in this group, and it is discussed as steroid 48 in Chapter 19. It is a powerful inhibitor of aromatase. [63-64] Formestan is also for sale in food supplements and it is on the market as a gel.
Oxymesterone is the 17a-methyl analogon of formestan. The anabolic properties of this steroid were already mentioned in the beginning of the sixties [65] and they are better than those of methyltestosterone. The androgenic side effects are a bit less, which results in a good ratio between anabolic and androgenic properties for this steroid.
Oxabolone is the 19-nor-analog of formestan. In Chapter 19 it is mentioned as steroid 46. Oxabolone is an anabolic steroid and does not inhibit aromatase. In Chapter 19 was already explained that the C19 oxidized intermediates (see also Scheme 2) may play a crucial role in the aromatase inhibition process. In 19-nor steroids there is no C19 methyl group and such intermediates can not be formed. [66] The anabolic properties of oxabolone have been investigated and patented already in the sixties by an Italian group. [67] Later esters and ethers of this steroid were patented again. [68]
Group 10. Chloro containing steroids
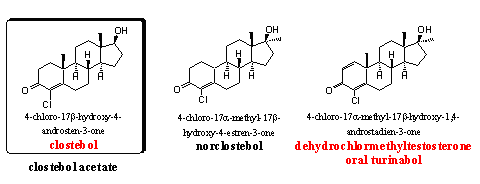
Clostebol-acetate is a mild orally active or injectable anabolic steroid. It has a favorable ratio between anabolic and androgenic properties, it does not aromatize and it is not toxic for the liver. It is a suitable steroid for women and for elder people.
Norclostebol is also a mild anabolic steroid with few androgenic side effects. Clostebol and norclostebol have been developed by the Italian Lab. Farmitalia Milan in the mid fifties [69], but also Syntex has investigated these steroids. [70]
Oral Turinabol became famous and notorious as the anabolic steroid that was used by sportsmen from the DDR. The steroid was already known in the beginning of the sixties and its good anabolic and modest androgenic properties were also known at that time. [71-72] Later, when the abuse by athletes from the DDR became apparent, the metabolism of oral turinabol was investigated. [73] After the fall of the DDR, oral turinabol disappeared, but Chinese and underground laboratories have reintroduced it on the black market.
Group 11. Steroids with a heterocyclic ring at ring A
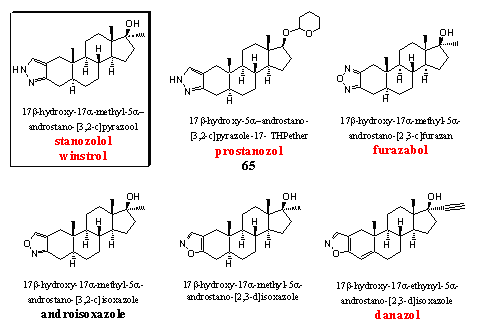
Steroids with a heterocyclic ring at ring A have been investigated at the end of the fifties and in the beginning of the sixties by Sterling-Winthrop. [74] [75] Stanozolol is the best known steroid in this group. Most of the steroids in this group do not have a D4 double bond and therefore they do not aromatize, they do have substantial androgenic side effects.
Stanozolol was already a well known and sought after anabolic steroid, but it became world famous when 100m winner Ben Johnson was said to be caught after using it in the Los Angeles Olympics in 1988. Stanozolol is a good orally active or injectable anabolic. Its activity is better than that of methyltestosterone but less than that of dianabol. The ratio between anabolic and androgenic activities has been investigated in different ways by several groups and the results are also different. An average of about 6 indicates that his ratio is a favorable one. A low dose of this steroid is also suitable for women.
Prostanozol has the same pyrazole ring at ring A as stanozolol, but the 17a-methyl group is absent here. Prostanozol is for sale as a food supplement and in advertisements it is called a prohormone. That is however not the case, it is a derivative of a normal anabolic steroid. The activity of the steroid is comparable with that of stanozolol. The C17 hydroxyl group is protected here as its THP ether. When this ether is absent, the steroid is not orally active. [74] Recently an olympic champion has been caught on the abuse of prostanozol. [76]
Furazabol is a heterocyclic steroid from Japan. [77] It is a relatively mild anabolic steroid with considerable androgenic side effects. From this steroid also a THP-ether derivative without a 17a-methyl group, was found in a food supplement (see Chapter 19, structure 66).
The isoxazole ring in androisoxazool is attached to ring A in a [3,2-c] manner. This means that the N-atom is situated on the place of the C3-carbonyl group. This anabolic can be taken orally and it is about one and a half time as active as methyltestosterone but its androgenic activity is only 20% of that of methyltestosterone. This means that it has a favorable ratio between anabolic and androgenic activities. The ratio between anabolic and androgenic activities is even better when the isoxazole ring is attached in a [2,3-d] manner at ring A. This means that the O-atom is situated on the place of the C3-carbonyl group. This steroid is depicted in the middle of the bottom row. It is not mentioned in the WADA list and also no trade name is known. The oral anabolic activity is nine times larger and the androgenic activity is four times smaller that of methyl testosterone. [75]
For danazol there is trade name and a place on the WADA list. In this steroid the isoxazole ring is also attached in a [2,3-d] manner to ring A. This steroid is used for treatment of endometriosis. It has mild anabolic properties although some people have different opinions. [1] An a-ethynyl group usually is not favorable for anabolic activity and a methyl or ethyl group in this position is better.
Many [3,2-c]pyrazole, [3,2-c]isoxazole and [2,3-d]isoxazole steroÔds have been synthesized and tested, with or without D4 double bond and with or without C17 a-methyl or ethyl group. [74] [75] Among them there were anabolic steroids with good activities but most of these have never reached the market.
Conclusions
The anabolic steroids in this chapter are the result of a long search of the pharmaceutical industry, for optimal and selective performing compounds. A high activity, in combination with a complete separation of anabolic and androgenic effects, was and still is the desired goal. This goal has not yet been reached today, but considerable progress has been made. The understanding of important factors in the interaction between ligand and receptor has increased enormously, especially from crystal-structure determinations of the ligand bonding domain of ligand-androgenreceptor complexes (see Chapter 7). This better understanding also has induced the search for compounds with completely different, non steroidal, structures. They hope to realize their goals easier and quicker with these so called SARMís (see Chapter 20).
Some general remarks can be made about the structure-activity profiles of anabolic steroids.
- In testosterone-Type steroids, the most common structural variation is reduction at C3 and oxidation and derivatization at C17. Aromatization is practically unavoidable in these steroids and fat and water retainment is the result. Reduction of the D4 double bond is also a normal metabolic transformation, which leads to dihydro testosterone-type steroids, which often show considerable androgenic side effects.
- 19-Nor-androstenes. The omission of the C19 methyl group in 19-nor androstenes usually increases the anabolic activity, aromatization does occur, but is not a serious problem. Reduction of the D4 double bond usually leads to less active steroids with less androgenic side effects.
- 17a-Methyl- and -ethyl-androstenes. The introduction of a C17-methyl or ethyl group has a positive effect on the anabolic activity. These steroids are orally active because metabolic transformations around C17 do not take place or are slowed down. Changes at C17 usually do not influence the reactivity of ring A and the normal metabolic reactions like aromatization and reduction of the D4 double bond remain possible. The metabolites than also show the corresponding side effects.
- The introduction of one or more methyl groups at C17, C7, C11 and sometimes at C1 and C2 generally has a positive effect on the anabolic activity of the steroid. [78] These so called porcupine steroids have a larger surface and a better Van der Waals interaction with the androgen receptor.
Dihydrotestosterone-type steroids often show considerable androgenic side effects but these are not always predictable.
- D1-Testosterone-type steroids. The D1 double bond often enhances the anabolic activity of steroids. Aromatization is not common (dianabol is an exception) and reduction of the D4 double bond does not occur. The androgenic activity of these steroids is therefore not modified and can be considerable.
- 19-nor androstenes with several double bonds usually are active to very active anabolic steroids. These flatter and more flexible steroids have an improved interaction with the androgen receptor. Aromatization and reduction of the D4 double bond does not occur, but androgenic side effects may be large.
- Steroids with an extra hydroxyl group at C4 The activity of steroids with an extra hydroxyl group at C4 depends on the structure of the rest of the steroid molecule.
- Steroids with a chloro atom at C4 The introduction of a chloro atom at C4 usually lowers the anabolic activity of the steroid, but the androgenic activity is mostly lowered more. The steroids are mild anabolics
- Steroids with a heterocyclic ring at ring A. The attachment of a heterocyclic ring at ring A enhances the anabolic activity. These steroids do not aromatize but the androgenic side effects are considerable.
- It is still rather unpredictable how a combination of structural changes ultimately will change the activity and selectivity of an anabolic steroid. Simple testing is the only way to find out and often experience will be superior to scientific knowledge.
[1] Duchaine D. Underground steroid handbook.
[2] Llewellyn W. Anabolics 2002.
[3] World Anti-Doping Agency (WADA) The 2007 prohibited list.
[4] Solveig H. and Hemmersbach P. Kjemi (2006) 66, 8-12.
[5] Bricout V. and Wright F. European Journal of Applied Physiology (2004) 92, 1-12.
[6] Velazquez I. and Alter B.P. American Journal of Hematology (2004) 77, 257-267.
[7] Koller M. and Ercoli A. Bollettino - Societa Italiana di Biologia Sperimentale (1952) 28, 1726-1729.
[8] De Ruggieri P. and De Giuseppe L. Il Farmaco; edizione scientifica. (1953) 12, 703-705.
[9] Arnold A. and Potts G.O. Acta Endocrinologica (1966) 52, 489-496.
[10] Saunders F.J. and Drill V.A. (searle) Proceedings of the Society for Experimental Biology and Medicine (1957) 94, 646-649.
[11] Partridge J.W.; Boling l.; de Wind L.; Margen S and Kinsell L.W. Journal of Clinical Endocrinology and Metabolism (1953) 13, 189-202.
[12] Brits patent GB 863662.
[13] Arnold A.; Potts G.O. and Beyler A.L. Journal of Endocrinology (1963) 28, 87-92.
[14] Stucki J.C.; Duncan G.W. and Lyster S.C. (Upjohn) Hormonal Steroids, Proceedings of the International Congress on Hormonal Steroids (1965) 2, 119-132.
[15] Campbell J.A. and Babcock J.C. (Upjohn) Hormonal steroids, Proceedings of the International Congress on Hormonal Steroids (1965) 2, 59-67.
[16] Herr M.E.; Hogg J.A. and Levin R.H. (Upjohn) Journal of the American Chemical Society (19560 78, 500-501.
[17] Marinosci A.; Peruzy A.D.; De Martino C. and Sabella G. Folia Endocrinologica (19600 13, 113-140.
[18] Kraft H.G. and Bruecker K. Artzneimittel Forschung (1964) 14, 326-330.
[19] Kraft H.G. and Kieser H. Artzneimittel Forschung (1964) 14, 330-335.
[20] Boris A.; Stevenson R.H. and Trmal T. Steroids (1970) 15, 61-71.
[21] Perrine J.W. Acta Endocrinologica (1961) 37, 376-384.
[22] Colton F.B. (Searle) US patent 2721871.
[23] Drill V.A. and Saunders F.J. (Searle) Hormones and aging process. Proc. Conf Harriman, New York (1955) 99-113.
[24] Overbeek G.A. and de Visser J. Ann. Endocrinol. (Paris) (1956) 17, 268-269.
[25] Dorfman R.I. and Kincl F.A. Endocrinology (1963) 72, 259-266.
[26] R.A. Edgren (Wyeth Labs) Acta Endocrinologica (1963) 87, 21.
[27] Campbell J.A.; Lyster S.C.; Duncan G.W. and Babcock J.C. Steroids (1963) 1, 317-324.
[28] Lyster S.C. and Duncan G.W. Acta Endocrinologica (1963) 43, 399-411.
[29] Szpitlfogel S.A. and cooperators patenten GB 811961 (1959), NL 89813 (1958), GB 91082 (1959), NL 95799 (1960) and US 3112328 (1963).
[30] Organon NV Dutch patent NL 6406797.
[31] Ruggieri P. de Italian patent IT 199611123 and IT 19630403.
[32] Vazques E.; Hinojosa C.; Guevara G.; Guerrero A.; Bruciaga V.; Llaca V.; Velasco S.; Vazques P. and Ortega L. Ginecologia y Obstetricia de Mexico (19630) 18, 549-567.
[33] Matscher R.; Lupo C. and Ruggieri P. de Bollettino- Societa di Biologia Sperimentale (1962) 38, 988-990.
[34] Ruggieri P. de; Gandolfi C. and Chiaramonti O. Bollettino- Societa di Biologia Sperimentale (1962) 38, 985-987.
[35] Ringold H.J. and Rosenkranz G, Journal of Organic Chemistry (1956) 21, 1333-1335.
[36] Ringold H.J.; Batres E.; Halpern O. and Necoechea E. (Syntex) Journal of the American Chemical Society (1959) 81, 427-432.
[37] Babcock J.C.; Campbell J.A. and Pederson R.L. (Upjohn) Danish patent DE 1117114.
[38] Ergogenics.
[39] Llewellyn W. Anabolics 2002, p. 48.
[40] Pappo R. (Searle, 1964) DE 1171425 (Priority US 19600517).
[41] Pappo R. and Jung C. US patent 3101349.
[42] Fennessey P.V.; Gotlin R.W.; Martin D.; Smith S. and Harrison L.M. Journal of Pharmaceutical and Biomedical Analysis (1987) 6, 999-1002.
[43] Fox M.; Minot A.N. and Liddle G.W. Journal of Clinical Endocrinology and Metabolism (1962) 22, 921-924.
[44] Lennon H.D. and Saunders F.J. Steroids (1964) 4, 689-697.
[45] Sheffield-Moore M.; Urban R.J.; Wolf S.E.; Jiang J.; Catlin D.H.; Herndon D.N.; Wolfe R.R. and Ferrando A.A. Journal of Clinical Endocrinology and Metabolism (1999) 84, 2705-2711.
[46] Schroeder E.T.; Zheng L.; Yarasheski K.E.; Qian D.; Stewart Y.; Flores C,; Martinez C.; Terk M. and Sattler F.R. Journal of Applied Physiology (2004) 96, 1055-1062.
[47] Zie Ergogenics.
[48] Weichert R. and Goedicke V. (Schering A.-G.) German patent DE1152103.
[49] Weichert R. and Caspar E. Chemische Berichte (1960) 93, 1710.
[50] Counsel R.E.; Klimstra P.D. and Cotton F.B. J. Org. Chem. (1962) 27, 248.
[51] Kincl F.A. and Dorfman R.I. Steroids (1964) 3, 109.
[52] Nutting E.F.; Klimstra P.D. and Counsel R.E. Acta Endocrinologica (1966) 53, 627 and 635.
[53] Ercoli A.; Gardi R. and Vitali R. Chemistry and Industry (London) (1962) 1284.
[54] Llewellyn W.C. US patent 2003 nr 641528.
[55] Galetti F. and Gardi R. Steroids (1971) 18, 39-50.
[56] Todd T. Journal of Sport History (1987) 14, 87-107.
[57] Fair J.D. Journal of Sport History (1993) 20, 1-24.
[58] Laboratorio Prodotti Braglia, British patent (1969) nr GB 1168931; French patent (1969) nr FR 6061.
[59] Vignau R.; Bucourt R.; Tessier J.;Costerousse G.; Nedelec L.; Gasc J-C.; Joly R.; Warnant J. and Goffinet B. Roussel UCLAF US patent 3 453 267.
[60] Perelman M.; Farkas B.; Fornefeld E.J.; Kraay R.J. and Rapala R.T. Journal of the American Chemical Society (1960) 82, 2402-2403.
[61] Nutting E.F. and Calhoun D.W. Searle Endocrinology (1969) 84, 441-442.
[62] Roussel-Uclaf Nederlands patent NL 6607609; German patent DE 1593307; British patent GB 1069709; French patent FR 1503984.
[63] Marsh D.A.; Brodie H.J.; Garrett W.; Tsai-Morris C,H,en Brodie A.M.H. Journal of Medical Chemistry (1985) 28, 788-795.
[64] Rowlands M.G.; Foster A.B.;Mann J.;Pietrzak B.;Wilkinson J. and Coombes Steroids (1987) 49, 371-382.
[65] Dorfman R.I. and Kincl F.A. Endocrinology (1963) 72, 259.
[66] Covey D.F. and Hood W.F. Molecular Pharmacology (1982) 21, 173-180.
[67] Camerino B.; Patelli B. and Seiaky R. Italian patent 19610210, US patent 3068247.
[68] Sal A. US patent 2003199466, US patent 2004002483.
[69] Sala G.; Baldratti G.; Ronchi R.; Clini V. and Bertazolli C. Sperimentale (1956) 106, 490-510.
[70] Ringold H.J.; Batres E.; Mancera O. and Rosenkranz G. (Syntex) Journal of Organic Chemistry (1956) 21, 1432-1435.
[71] Schubert A.; Stachowiak D.; Onken D.; Specht K.; Barnikol-Oetler K.; Bode E.; Heller K.; Polnert S Schwartz S and Zepter R. Pharmazie (1963) 18, 323.
[72] Dorner G.; Stahl F. and Zabel Endocrinologie (1963) 45, 121.
[73] Musshof F.; Daldrup T. and Ritsch M. Journal of Forensic Sciences (1997) 42, 1119-1125.
[74] Clinton R.O.; Manson A.J.; Stonner F.W. Neumann H.C.; Christiansen R.G.; Clarke R.L.; Ackerman J.H.; Page D.F.; Dean J.W.; Dickinson W.B. and Carabateas C. Journal of the American Chemical Society (1961) 83, 1478-1491.
[75] Manson A.J.; Stonner F.W. Neumann H.C.; Christiansen R.G.; Clarke R.L.; Ackerman J.H.; Page D.F.; Dean J.W.; Phillips D.K.; Potts G.O.; Arnold A.; Beyler A.L. and Clinton R.O. Journal of Medicinal Chemistry (1963) 6, 1-9.
[76] Ergogenics.
[77] Shimizu M.; Ohta G.; Veno K.; Takegoshi T.; Oshima Y.; Kasahara A.; Onodera T.; Mogi M. and Tachizawa H. Chem. & Pharm. Bull. (Tokyo) (1965) 13, 895.
|
|