Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 15/11/2008
19. Steroids in Designer Supplements
Aede de Groot, Willem Koert
In the former chapters we have paid attention to natural anabolics, synthetic anabolics, prohormones, hormone derivatives and designer steroids. In this chapter we will discuss the anabolic steroids in food supplements. Food supplements that are tuned with synthetic steroids are also known as designer supplements
Steroids in designer supplements have not been “designed” to avoid detection in doping controls, but more to avoid violation of patents or restrictions by law. On the analogy of the definition of designer steroids, designer supplements can be described as food supplements which contain steroids that are not forbidden by law. Mostly these steroids never have been tested on humans. Most of the knowledge about their anabolic and possible side effects originate from animal experiments and from users.
In 2006 and 2007 we have searched designer supplements on the internet and we have tried to find out which steroids they contain. This has not been a simple task. The nomenclature on the labels often is old fashioned, sloppy, sometimes purposely unclear or simply absent. The list does not mention all the presently available designer supplements. Continuously new products appear on the market, and other disappear. Sometimes the new supplements contain new steroids, sometimes old ones reappear under a different name. However, this list gives a good impression about how producers of designer supplements compose their products and advertise them.
We have found 74 different steroids in designer supplements and they are presented here in nine different groups. This subdivision is based on their chemical structure and on their type of activity.
The prohormones and hormone derivatives of testosterone and dihydrotestosterone are places in the first two groups. Since many of these two steroids also have 19-nor and/or 17a-methyl analogs, these compounds are also placed in the same groups one and two.
Steroids with extra double bonds, hydroxyl groups and carbonyl groups, together with their derivatives and the corresponding methyl analogs are placed in the next groups.
Finally there is a group with chloro or bromo atoms in the molecule and a rest group of steroids that could not be placed easily in one of the other groups.
This approach allows a reasonably clear subdivision in the following 9 groups:
1. Prohormones of testosterone and their derivatives.
These testosterone like steroids are oxidized or reduced at C3 or C17. The corresponding 19-nor and
17a-methyl analogs are also placed in this group.
2. Prohormones of dihydrotestosterone and their derivatives.
These dihydrotestosterone like steroids have a 5a H-atom and are oxidized or reduced
at C3 or C17. The corresponding 2a- and 17a-methyl analogs
are also placed in this group.
3. D1-testosterone, its prohormones and derivatives.
These steroids are oxidized or reduced at C3 or C17, and the corresponding 17a-methyl analogs
are also placed in this group.
4. Steroids with more then one double bond and their derivatives.
Steroids that are oxidized or reduced at C3 or C17 and the corresponding 19-nor and
17a-methyl analogs are also placed in this group.
5. Steroids with an extra hydroxyl group at C4 and their derivatives.
Steroids oxidized at C17 and a 17a-methyl analog are also placed in this group.
6. Steroids with an extra hydroxyl or carbonyl group at C6 and their derivatives.
These steroids all have a carbonyl group at C17. An extra double bond may be present as well.
7. Steroids with an extra hydroxyl or carbonyl group at C7 and their derivatives.
Steroids that are oxidized or reduced at C17 and a 19-nor analog are also placed in this group.
8. Steroids with a chloro or bromo atom.
9) Other steroids.
Group 1. Prohormones of testosterone and their derivatives
These testosterone like steroids are oxidized or reduced at C3 or C17. The corresponding 19-nor and 17a-methyl analogs are also placed in this group.
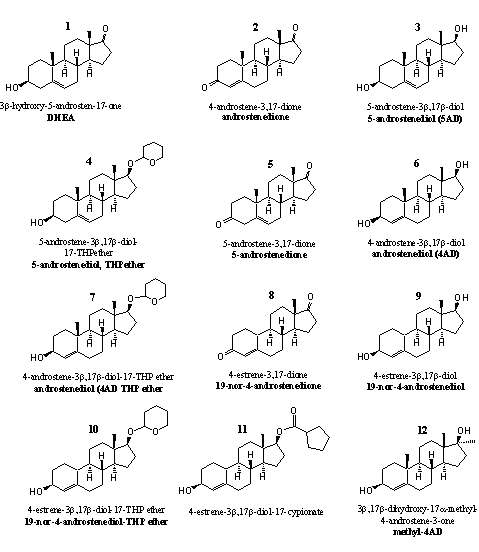
Figure 1
Steroids 1, 2 and 3 on the upper row are intermediates in the biosynthesis of testosterone (see Chapter 11). They are prohormones of testosterone. The adrenaline cortex produces large quantities of DHEA and DHEA-sulfate. In muscle tissue, in the skin and in the prostate, enzymes convert DHEA into testosterone and dihydrotestosterone. [1] [2]
Most of the prohormones of testosterone are mentioned on the list of prohibited steroids of the American Anabolic Steroid Control Act of 2004. DHEA is not on this list, although researchers have concluded that DHEA has anabolic properties, which do not differ essentially from those of dihydrotestosterone and THG. [2]
The Anabolic Steroid Control Act does mention the two other prohormones androstenedione 2 and 5-androstenediol 3. However, DHEA is mentioned on the list of the world doping authority WADA.
Users of DHEA consider it in the first place as an anti-aging-compound and not as an anabolic steroid. Producers have introduced androstenedione and androstenediol as anabolics but users were mostly disappointed by their effectiveness. Both hormones proved to be anabolic only at extremely high doses and then showed serious side effects. Especially their conversion into estradiol caused problems.
Compound4 is a prohormone and derivative in one. In the body the THP ether should be removed to form 5-androstenediol, which then can be transformed by enzymes into testosterone.
Steroid 5 is also an intermediate in the biosynthesis of testosterone. The enzyme 3b-hydroxy-steroid dehydrogenase/D5-D4-isomerase, or 3b-HSD, first oxidizes the C3-hydroxyl group to a carbonyl group to form DHEA 1. The same enzyme then catalyzes the shift of the double bond from position 5 to position 4 under formation of androstenedione 2. Finally testosterone is obtained when the enzyme 17b-dehydrogenase reduces the carbonyl group at C17 in dione 2 to a hydroxyl group.
Steroid 6 at the right on the second row is a prohormone for testosterone but not an intermediate in its biosynthesis. [3] [4] It has already been mentioned above that in the biosynthesis of testosterone the hydroxyl group at C3 is oxidized first and the shift of the double bond takes place next. In steroid 6 the double bond is already in the desired 4 position and a 3b-dehydrogenase just has to oxidize the hydroxyl group at C3 to a carbonyl group. The two conversions take place here in a reversed sequence. Steroid 7 is the THP-ether derivative of 6.
Steroids 8-11 are prohormones of nandrolone. In steroid 8, a 17b-dehydrogenase has to reduce the carbonyl group at C17 to a hydroxyl group to get nandrolone. Users of prohormone 8 excrete the same metabolites as users of nandrolone itself, which indicates that the conversion of 8 into nandrolone indeed takes place. [5]
In steroid 9 a 3b-dehydrogenase has to oxidize the hydroxyl group at C3 to a carbonyl group. Positive effects of moderate doses of norandrostenedione 8 and norandrostenediol 9 on resistance trained athletes have not been found. [6]
An advantage of 19-nor steroids is that their 5a-reduced analogs show a reduced binding with the androgen receptor in comparison with dihydrotestosterone. Consequently, many 19-nor steroids show less androgenic side effects. They are milder for the prostate and have SARM-like properties. Recently such SARM-like properties have been shown for 19-nor-4-androstene-3b,17b-diol 9. [7]
Steroids 10 and 11 are a THP-ether and an ester derivative of 9 respectively.
Steroid 12 on the bottom row is the 17a-methyl-analog of 6. Russian researchers have published some animal studies in which an anabolic effect has been concluded. Oxidation of the hydroxyl group at C3 leads to the anabolic steroid 17a-methyltestosterone, and steroid 12 is its prohormone.
Users of anabolic steroids are usually not very enthusiastic about 17a-methyltestosterone. Aromatization easily leads to 17a-methylestradiol, a strong estrogenic steroid. Besides, 17a-methylsteroids easily may cause liver damage.
|
Addition: PheraPlex Not described in this chapter, but marketed as the active principle in designer supplements as PheraPlex and Ergomax LMG, is desoxymethyltestosterone. Scientists also call it madol or 17a-methyl-5a-androst-2-en-17b-ol. Before it was brought on the market by supplement producers, it has been used by chemical sporters, because it was not detected in routine doping tests. They bought it from rogue chemists who found it in elder literature. We have already described madol in Chapter 18.
|
|
Addition: 4AD returns In 2004 several prohormones and steroids in food supplements were forbidden by the American government. Among them were the prohormones of testosterone. However, in the Anabolic Steroids Control Act almost no steroids with a C17 carbonyl group are mentioned. Supplement producers gratefully use this omission, and have marketed 4-androstene-3b-ol-17-one as a testosterone precursor.
|
Group 2. Prohormones of dihydrotestosterone and their derivatives
These dihydrotestosterone like steroids have a 5a H-atom and are oxidized or reduced at C3 or C17. The corresponding 2a- and 17a-methyl analogs are also placed in this group.
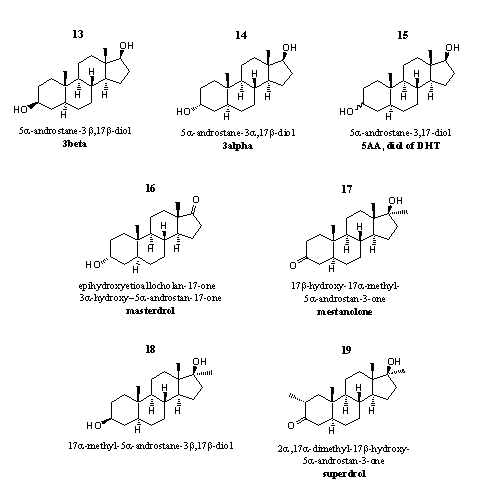
Figure 2
The steroids in this group do not have a double bond in the 4 position, which means that one of the essential conditions for aromatization of ring A is lacking (see Chapter 14). Users of these steroids will not suffer from gynecomastia. On the other hand, androgenic side effects probably will occur more often.
It is not always clear whether the steroids in this group are intended as prohormones or as active steroids themselves. Dealers and producers usually advertise them as prohormones.
The active target hormone in which these prohormones should be converted by enzymes in the body can not be testosterone or 17a-methyl testosterone. This would require the introduction of a double bond in the 4 position, but there are no enzymes known that can perform that transformation. In chapter 11 is explained that the enzyme 5AR can remove the double bond from testosterone to form dihydrotestosterone, but the reverse reaction does not occur.
Steroids 13-15 are prohormones for dihydrotestosterone. 3b-Dehydrogenases convert these steroids into dihydrotestosterone. It may be expected that these steroids will have considerable androgenic side effects. The hydroxyl groups in these steroids will be easily converted into a glucuronate or a sulfate and they will be quickly excreted.
Steroid 13 is present in designer supplements that are supposed to create a so-called "alpha-effect" in male users. The term "alpha-effect" originates from ethology and describes the behaviour of the leading male in a group of primates. A detail, which is mentioned surprisingly few in advertising, concerns the fact that in test tube experiments, steroid 13 binds to the estradiol receptor.
Steroid 14 plays a role in the addiction to testosterone. In animal experiments, testosterone proves to be less addictive when its enzymatic transformation into 14 is blocked. This compound causes an euphoric effect. The properties of 14 have been reviewed recently. [8]
Steroid 15 is the active compound in the designer supplement 5AA. It is a mixture of the 3a- and the 3b-hydroxyl compounds 13 and 14, mentioned above. This mix should increase aggression so that users can exercise more intensively. The producer claims that these compounds will help to reduce fat deposits through their androgenic action.
It has taken some trouble to discover the structure of steroid 16, the active steroid in the designer supplement Liquid Masterdrol. [9] The producer calls it epihydroxyetioallocholan-17-one. Etioallocholan is an old fashioned name for the androstane skeleton (see Chapter 5), epi means that the hydroxyl group is orientated differently. b-Hydroxy is the normal orientation in this old nomenclature, which means that in this case the hydroxyl group is in the a position. According to the advertising of the producer, this prohormone ultimately will be converted into stanolone. In principle this is possible because stanolone is the old fashioned name for dihydrotestosterone. To realize the conversion of 16 in dihydrotestosterone, a dehydrogenase should reduce the carbonyl group at C17 to a b-hydroxyl group, and another dehydrogenase should oxidize the hydroxyl group at C3 to a carbonyl group.
Maybe steroid 14 is an intermediate in this metabolic conversion of steroid 16 and in this way the claimed effects like "increased focus and intensity" may be realized. This prohormone should be beneficial for muscle definition and power. It is however also known that the end product dihydrotestosterone is responsible for androgenic side effects.
Steroid 17 is the 17a-methyl-analog of dihydrotestosterone. About ten commercial names are known for this steroid, among them are mestalone, mestanolone and methylandrostanolone. Steroid 17 itself is an anabolic steroid. [10-12] In the communistic DDR it was used as a doping agent, although the safety of mestalone had not been investigated.
Doping hunters have detected steroid 18 often as a metabolite of anabolic steroids. Steroid 18 is a metabolite of 17, but also of dianabol and 17a-methyltestosterone. Not much is known about its anabolic properties. Enzymes can oxidize the hydroxyl group at C3 and in this way steroid 18 is a prohormone for mestanolone 17.
Steroid 19 is marketed under the name superdrol. Some producers call it a prohormone but that is not correct. Superdrol itself is an active anabolic steroid. Superdrol resembles drostanolone (masteron). The difference is that in drostanolone there is no 17a-methylgroup at the five membered ring and in superdrol there is one. The powerful anabolic effect and the low androgenic activity of superdrol has been established by researchers of Syntex already in 1959. [14] The most probable metabolic transformation of superdrol is the reduction of the C3-carbonyl group to an a- and/or b-C3-hydroxyl group, and such a transformation usually diminishes the anabolic activity of a compound.
Users have learned to know superdrol as a steroid that may be harmful for the liver. After the appearance of superdrol on the market as a designer supplement, physicians several times have noticed liver damage and kidney damage. [14a]
|
Addition: Viratase Not mentioned in this chapter, but once marketed as a designer supplement, is the dione analog of dihydrotestosterone: 5a-androstan-3,17-one. Supplement company Molecular Nutrition has marketed this compound as Viratase. Molecular Nutrition also possessed the American patent on the use of this compound as a prohormone for dihydrotestosterone. [14b] That patent also covers the use of steroids 13 and 14, but not that of steroid 16, the mysterious substance in Liquid Masterdrol.
|
Group 3. D1-Testosterone, its prohormones and derivatives
These steroids are oxidized or reduced at C3 or C17, and the corresponding 17a-methyl analogs are also placed in this group.
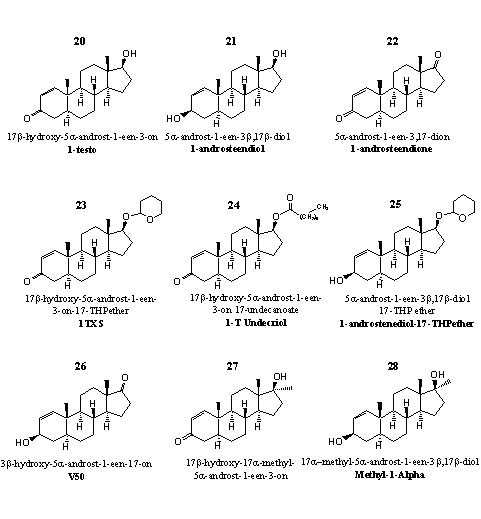
Figure 3
In these steroids there is no double bond in position 4, which is one of the essential preconditions for aromatization of ring A. Gynecomastia will not be a side effect in the use of these steroids.
1-Testosterone (1-Testo), steroid 20, is the leading steroid in group 3. Supplement producers advertize 1-testosterone sometimes as prohormone, but that is not correct. In which real hormone enzymes have to convert 1-testosterone? Reduction of the double bond will lead to dihydrotestosterone and that is not an anabolic but an androgenic steroid. An additional double bond at position 4 will lead to the anabolic steroid boldenone, but it has already been remarked in this chapter that this enzymatic reaction does not take place in our body. Recent investigations have shown that 1-testosterone itself is a potent anabolic steroid. Its anabolic and androgenic properties are comparable with those of testosterone propionate. [15] Some preparations with 1-testosterone have been marketed as a trans dermal gel.
Users describe 1-testosterone as a powerful anabolic steroid. The main side effect on short notice is a lethargic mood, sometimes a bit like a depression, and a diminished appetite. Long term side effects are not yet clear.
Producers have marketed several prohormones for 1-testosterone. The prohormones 21 and 22 have been patented by Pat Arnold. [16] [17] In steroid 21 a 3b-dehydrogenase has to oxidize the hydroxyl group at C3 to a carbonyl group. In steroid 22 a 17b-dehydrogenase has to reduce the carbonyl group at C17 to a hydroxyl group.
Steroids 23 and 24 are the THP ether and the ester derivative of 1-testosterone. Steroid 25 is the THP-ether derivative of 22, and in this way it is a prohormone and a derivative of 1-testosteorne in one.
Steroid 26 is also intended as a prohormone for 1-testosterone, but to reach that goal two enzymatic reactions have to take place. Enzymes have to oxidize the C3-hydroxyl group to a carbonyl group and to reduce the C17-carbonyl group to a hydroxyl group.
Steroid 27 is the 17a-methyl-analog of 1-testosteron. It is a powerful anabolic steroid that has been investigated already in the sixties of the former century. [18-20] In a patent of CIBA the anabolic activity is claimed to be twenty times that of 17a-methyltestosterone while the androgenic activity should be only half as large. [21] Earlier investigations are a bit more modest and mention an anabolic activity half as large and an androgenic activity of one quarter of that for testosterone propionate. [20]
After the appearance of steroid 27 on the market, health authorities have mentioned liver damage in users of designer supplements with this steroid as active ingredient.
The chemical structure of the active ingredient in the designer supplement Methyl-1-Alpha is a bit mysterious. The producer calls it methyl-1-etiocholenolol, which does not give information about the place of the methyl group, the double bond and the oxidation state of the molecule. We suppose that this name stands for structure 28. Researchers of Searle have published about this compound already in 1962. [20] The anabolic activity is about the same as that of the corresponding C3-carbonyl compound 27, but the androgenic activity is twice as large. It seems reasonable to consider steroids 27 and 28 as mutual prohormones, each with its specific anabolic and androgenic activity.
Group 4. Steroids with more then one double bond and their derivatives
Steroids that are oxidized or reduced at C3 or C17 and the corresponding 19-nor and 17a-methyl analogs are also placed in this group.
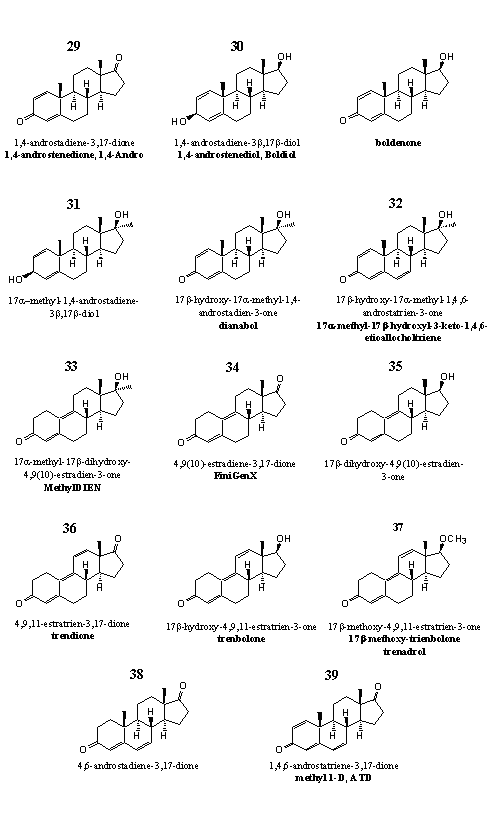
Figure 4
The steroids in Group 4 do have similarities in chemical structure, but their physiological activities are diverse. Producers and sellers often advertize their new steroids as compounds that are very similar to well known steroids but with a much better activity. Such remarks should be looked upon with some reserve. Similar types of steroids may have completely different physiological properties.
Steroids 29 and 30 are prohormones of the anabolic steroid boldenone, the structure of the latter is added in the figure. In steroid 29 a 17b-dehydrogenase has to reduce the carbonyl group at C17 to a hydroxyl group, in steroid 30 a 3b-dehydrogenase has to oxidize the hydroxyl group at C3 to a carbonyl group. 1,4,-Androstenedione 29 is patented as prohormone. [22]
Research has revealed that the metabolites, excreted after the use of quinbolone, the 17-cyclopentenyl-enolether of boldenone (see Chapter 17), 1,4-androstenedione 30 and boldenone itself, show a close similarity. This is a strong indication that quinbolone and androstenedione are converted by enzymes in the body to boldenone. [23] We have not found data about the biological activity and the metabolism of steroid 30 (boldiol). However, we assume that enzymes quickly will oxidize the C3 hydroxyl group to a carbonyl group and in this way convert it into boldenone.
The same is true for steroid 31, the 17a-methyl-analog of 30. Steroid 31 is intended as a porhormone of the well known anabolic steroid dianabol, also displayed in the figure above. Also in steroid 31 a 3b-dehydrogenase has to oxidize the hydroxyl group at C3 to a carbonyl group to convert it into dianabol. Steroid 31 does have a CAS-number, but not much can be found about this compound in the literature. Even publications about its synthesis are absent.
Not much is known about the biological activities of steroid 32. It is a metabolite of dianabol, and has been detected in the urine of users of dianabol. [26] Steroid 32 is also a side product in the synthesis of dianabol from methyltestosterone, and the separation of the desired product dianabol, from the sideproduct 32 is rather difficult. [27] Therefore, analytical chemists that analyze excretion products of dianabol, should be very careful. They have to control beforehand that no steroid 32 is present in the administered dianabol. Supplement producers also put steroid 32 in products that have to inhibit the production of estradiol in the body, or that have to reactivate the natural production of sex hormones after an anabolic cycle.
Steroid 32 is probably the most active component in the designer supplement Jungle Warfare. Analyses by a recognized laboratory showed that this supplement contains a mixture of several steroids in substantial quantities. [30] One of them could be dianabol.
Steroid 33, the active principle in designer supplement MethylDien and M-Dien, already has been synthesized and tested by researchers of Eli Lilly [28] in 1960, and the exceptionally high oral anti-estrogenic activity of this steroid was noticed. The same steroid has been investigated later by Searle. [29] They found an androgenic activity five to seven times higher than that of 17a-methyltestosterone. The anabolic activity was estimated about eighty times higher then that of 17a-methyltestosterone. Researchers of Roussel-Uclaf have described steroid 33 as a hormone with a high affinity for the progesterone receptor. [27a] Together with its androgenic properties, this can be an explanation for the anti-esterogenic activity of steroid 33. The steroid inhibits the endogenous production of steroid hormones and in this way also the concentration of estradiol is lowered.
Nothing about the biological activities of steroid 34 could be found in the literature. The steroid is mainly described as a useful intermediate in the production of other steroids. A dealer of a designer supplement with steroid 34 as active ingredient, claims in his advertisement: "Estra-4,9-diene-3,17-dione can easily be converted to trenbolone through organic chemical reactions. This is indeed possible but only in a chemical laboratory. We do not believe that the conversion of estra-4,9-diene-3,17-dione into trenbolone will take place in the body. The reduction of the C17-carbonyl group to a 17b-hydroxyl group is feasible but the introduction of the third double bond between C11 and C12 as never been noticed and is most improbable.
Steroid 34 will not be a prohormone for trenbolone as suggested by the producer. At the best, a steroid with one double bond less, 17b-hydroxy-4,9(10)-estradiene-3-on 35 will be produced in the body. This steroid has been tested by the same researchers that have made and tested steroid 33. [29] Steroid 33 has a 17a-methyl group and is more active then steroid 35, where this methyl group is lacking. The anabolic and androgenic activity of 35 is approximately the same as that of 17a-methyltestosterone.
We come back for a moment to the text in the advertising for steroid 34. "Estra-4,9-diene-3,17-dione molecule is literally the closest thing to trenbolone on the market." We did not check this, but we do have a suggestion for the producers of designer supplements. Why not use trendion 36? This steroid is even closer to trenbolone. A 17b-dehydrogenase can reduce 36 and really transform it into trenbolone.
The methyl ether of trenbolone 37 is marketed as a deisgner supplement under the names trenadrol and methoxytren. However an analysis of the supplement showed that no methoxy group was present in the active ingredient. The supplement just contained trenbolone. [30]
Aromatase inhibitors
Steroids 38 and 39 do have structural similarity with the other steroids in group 4, but their biological activity is completely different. These two steroids are aromatase inhibitors. This means that they form complexes with the enzyme aromatase and not with a steroid receptor. In Chapter 9 we have already discussed the differences between enzymes and receptors.
Not just the steroids 38 and 39 are aromatase inhibitors. Also part of the steroids in the groups 5 and 6 and the 6-bromo-steroid 61 from group 8 inhibit aromatase. They can do that as a competitive inhibitor or as a suicide substrate.
A competitive inhibitor competes with the natural substrate to form a complex with the enzyme. When more of the inhibitor is present then this steroid wins the competition and the natural substrate or an administered anabolic steroid can not interact with the enzyme anymore. In such cases these steroids are not converted into an aromatic steroid. When lower concentrations of the inhibitor are present, the natural steroidal substrate or the administered anabolic steroid still can form complexes with the enzyme in a restricted way and it will be transformed in the normal way into an aromatic steroid.
A suicide substrate binds so strongly to the enzyme that the complex does not dissociate after the reaction. The enzyme is blocked permanently and new substrates can not enter anymore and are not aromatized.
Steroid 38 is a competitive inhibitor; steroid 39, steroids 40 and 41 from group 5, steroids 49 and 52 from group 6 and the 6-bromo-steroid from group 8 61 are suicide substrates.
Aromatase inhibitors are useful for users of high doses of anabolic steroids that are susceptible to aromatization. These users may have to deal with gynecomastia. The structural conditions for aromatization have been discussed already in Chapter 14. There we have shown also the structural adaptations that can be brought into the steroids to prevent aromatization. Examples are also shown in groups 1 and 2 of this chapter. There are however steroids, steroid derivatives and prohormones with a high tendency for aromatization. Testosterone itself and its prohormones, and androstenedione as good examples.
Four structural changes can be brought into androstenedione to convert it from a natural substrate into a suicide substrate of aromatase (see Scheme 1). [31]
1) The introduction of a double bond between C1 and C2 (Group 4). An example is steroid 39 or ATD.
2) The introduction of a hydroxyl group at C4 (Group 5). An example is steroid 40 or Formestan.
3) The introduction of a carbonyl group at C6 (Group 6) An example is steroid 49 or 6-OXO.
4) The introduction of a bromo-atom at C6 (Group 8). An example is steroid 61 or Hyperdrol.
The enzyme-substrate complex of the steroids mentioned above do not dissociate anymore because a really strong covalent chemical bond is formed between substrate and enzyme. It is not yet completely clear why this happens with these steroids and not with the natural substrates or with many other synthetic anabolic steroids. It does however illustrate again that changes in the chemical structure of a steroid can have severe consequences for its biological activity.
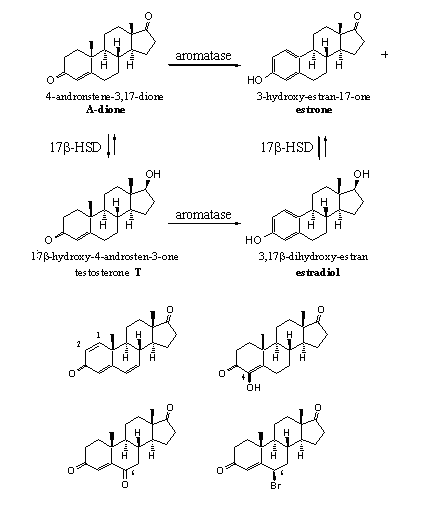
Scheme 1
Users of designer supplements are especially familiar with aromatase inhibitors in post cycle therapies or PCT’s. Producers advise to use PCT steroids to reactivate the endogenous production of testosterone after an anabolic cycle. PCT steroids lower the estradiol concentration in the blood and in this way stimulate the hypothalamus and the pituary to produce the necessary hormones to activate the testes. We will not comment on the usefulness of androgenic aromatase inhibitors for PCT, and also not on the question whether the supplements that are marketed for this purpose are really effective.
Researchers discovered already in 1973 that steroid 38 is a good inhibitor for the enzyme aromatase. [32] It is a competitive inhibitor and the structure-activity relations of substituted derivatives of this steroid have been investigated. [33-35] None of these structural variants of steroid 38 has appeared on the market, probably because better alternatives became available.
Steroid 39 is the well known aromatase inhibitor ATD. It is probably a suicide substrate. [31] [36] Other biological effects are claimed in patents. [37] [38]
Group 5. Steroids with an extra hydroxyl group at C4 and their derivatives
Steroids oxidized at C17 and a 17a-methyl analog are also placed in this group.
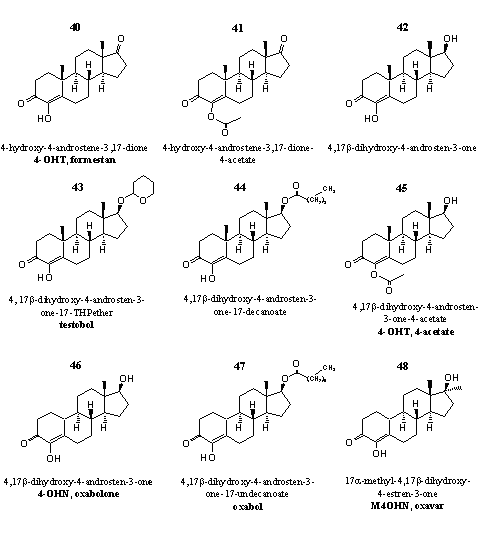
Figure 5
The leading steroid in this group is 4-hydroxy-3-androstene-3,17-dione (Formestan) 40. This compound is also marketed as a gel that can be applied on the skin. Formestan 40 and its corresponding 4-acetate 41 are powerful aromatase inhibitors [39] [40]. Both compounds are suicide substrates. [31] [36]
The 17b-hydroxy analog of 40, 4-hydroxytestosterone 42 is a weaker aromatase inhibitor then 40. [32] Doping hunters consider 42 more as an anabolic steroid [41], and it is placed on the doping list of the WADA. [42] Patents of American producers of designer supplements with this ingredient claim effects such as lowering of the estrogen level in elder men [43] and increase of athletic capabilities. [44]
Steroids 43, 44 and 45 are the THP-ether, the decanoate ester and the 4-acetate of 4-hydroxytestosterone, respectively.
The 19-nor-steroids 46-48 on the bottom row are not aromatase inhibitors. The reason for this may be that these steroids can not be converted into an intermediate that is crucial for the whole process of aromatization and its inhibition. This intermediate is the steroid with the C19 methyl group already oxidized to a carbonyl group (see Chapter 14, Scheme 2). When in this intermediate a 4-hydroxy, a D1,2-double bond, a 6-carbonyl group or a 6-bromo atom is present, a strong covalent enzyme bound product is obtained, which does not leave the catalytic cavity of the enzyme. In this way the enzyme is blocked completely for further catalytic action. In the 19-nor steroids there is no 19-methyl group that can be oxidized and therefore this crucial intermediate can not be formed. [45]
These 19-nor steroids have been investigated and patented already in the beginning of the sixties of the former century by an Italian group. [46] Their anabolic properties have been mentioned there, but did not attract much attention. Esters and ethers of these steroids have been patented later by American producers of designer supplements. [47]
Steroid 47 is an ester of 46, steroid 48 is the 17a-methyl-analog of 46.
Group 6. Steroids with an extra hydroxyl or carbonyl group at C6 and their derivatives
These steroids all have a carbonyl group at C17. An extra double bond may be present also.
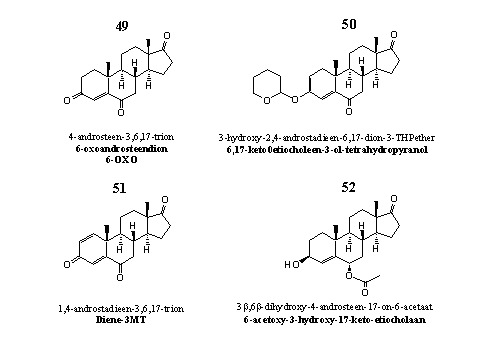
Figure 6
The leading steroid in group 5 is 6-OXO or 4-androstene-3,6,17-trione 49. Also this steroid is a suicide substrate and a powerful aromatase inhibitor. Also with 6-OXO, the C19-carbonyl intermediate is involved in the inhibition process. [48] [49] In 2005 Pat Arnold patented steroid 49 as a compound that stimulates the bodies own testosterone production. [50]
The hypothalamus stimulates via the Hypothalamus-Pituary-Testes signaling system (see Chapter 20) the testes to produce more testosterone when lower concentrations of androgens, estrogens and gestagens circulate in the blood. A lower concentration of estrogens thus stimulates the testes to produce more testosterone. Administration of anti-estrogens to men increases their testosterone level and that is what also happens with 6-OXO. The higher testosterone level on its turn, causes an increase of the estradiol level, according to a recent study. [51]
Steroid derivative 50 is the THP-ether of the enol-form of the C3-carbonyl-analog of steroid 49. Both structural elements, the THP-ether and the enol-ether are not stable in the acidic environment of the stomach. There derivative 50 will hydrolyze quickly to 6-OXO 49. Steroid 50 is one of the active ingredients in the designer supplement Novedex XT, together with ATD. Investigations, financed by the producer, have confirmed that the testosterone level is increased by Novedex XT. [52]
Steroid 51 is for sale as aromatase inhibitor. However, we have not found any publication in the literature to substantiate this claim.
Also steroid 52 can be found in designer supplements and in the advertising the aromatase inhibiting properties of this steroid are mentioned. Also in this case no confirmation for that claim could be found in the scientific literature. The corresponding C3-carbonyl compounds have been investigated extensively for their aromatase inhibiting properties. [53] The investigators conclude that the 6a-compounds are more active then the corresponding 6b-derivatives.
Group 7. Steroids with an extra hydroxyl or carbonyl group at C7 and their derivatives
Steroids that are oxidized or reduced at C17 and a 19-nor analog are also placed in this group.
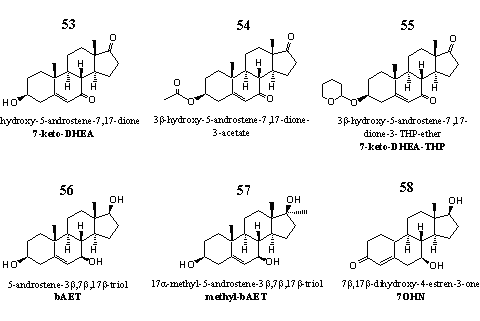
Figure 7
Most steroids in this group have a biological activity that differs from the steroids in the other groups. They stimulate the fat metabolism [54], and this property has attracted the attention of supplement producers. Several preparations with 7-keto-DHEA 53 as active ingredient have been patented [55-57]. Steroid 54 is the acetate, steroid 55 the THP-ether of 7-keto-DHEA.
Steroid 56 is a metabolite of 7-keto-DHEA, but it also can be considered as its prohormone. This steroid plays a role in the immune system of the body. [58] The 17a-methyl-analog 57 is patented as an immuno stimulant. [59]
Steroid 58 is unknown in the scientific literature and its biological effects are also unknown. Steroid 58 has no structural similarities with other steroids in this chapter.
|
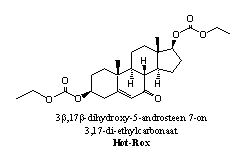
Addition: Hot-Rox The active steroid in Hot-Rox, a designer supplement of Bio-Test, resembles the steroids 56 and 53. The latter two steroids can be considered as a derivative and a prohormone for the Hot-Rox steroid, respectively. After hydrolysis of the carbonate esters and reduction of the carbonyl group at C7, steroid 56 is obtained. One also may consider the Hot-Rox steroid as a prohormone for 7-keto-DHEA 53. In that case, esterases also have to hydrolyze the carbonate esters. After that, the C17 hydroxyl group has to be oxidized to a carbonyl group to obtain 53.
|
Group 8. Steroids with a chloro or bromo atom
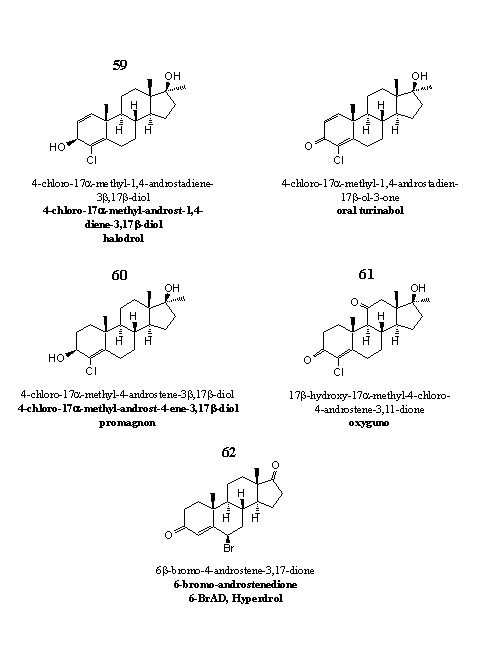
Figure 8
Steroids 59 and 60 are old favorites, and at the same time they are not. Doping experts are familiar with a steroid like 59 with a carbonyl group at C3 instead of a hydroxyl group. That steroid is known as Oral Turinabol, and in former times it was used intensively by sportsmen from the DDR.
Supplement producers have marketed steroid 59 as Halodrol. It is a prohormone for Oral Turinabol. The hydroxyl group at C3 can be easily oxidized by dehydrogenases to a carbonyl group. The metabolism of Oral Turinabol in humans has been investigated [60], but very little is known about Halodrol in the scientific literature.
Steroid 60 can be considered as a prohormone for 17a-methyl-clostebol. [61] Enzymes in the body just have to oxidize the C3-hydroxyl group to a carbonyl group. The advertisement claims a higher anabolic activity for the 17a-methyl-analog in comparison with the original molecule. This claim could not be substantiated with data from the scientific literature. Also nothing was found about the possible liver toxicity of steroid 60.
Steroid 61 has appeared on the market in 2007 as Oxyguno. Analysis showed that designer supplements with 61 as active ingredient actually contain two compounds: the 11-oxo and its 11b-hydroxy-analog. Experiments with animals from the sixties revealed that the androgenic activity of the 11-oxo-analog 61 is 7% of that of testosterone, but its anabolic activity is 850% of that of testosterone. The 11b-hydroxy-analog of this steroid has been used by sportsmen in the DDR. In documents from the archives of the secret service of the DDR this anabolic is indicated as Substanz XII.
The bromo containing steroid 62, 6-bromo-androstenedione, is marketed under the name Hyperdrol. It is not clear whether the product contains a 6a- or a 6b-bromo atom. Both steroids are aromatase inhibitors. [62]
Group 9. Other steroids
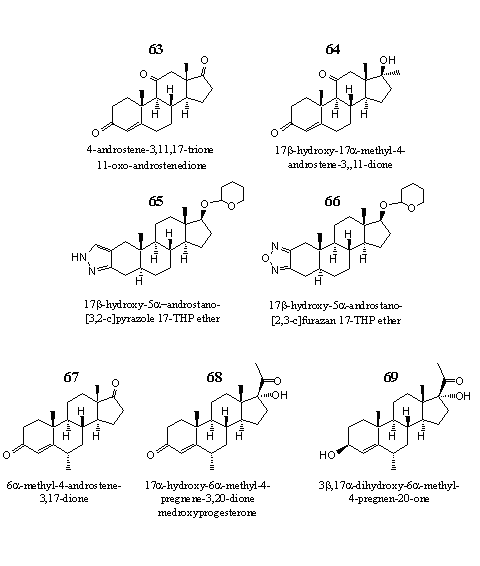
Figure 9
In this last group we have put together seven steroids that could not be placed easily in one of the other groups. Steroid 63 is an 11-oxo steroid, which is marketed with the name 11-OXO. The producers of 11-OXO do not claim an anabolic effect for this compound. Instead it should inhibit the biosynthesis of the catabolic steroid cortisol in the body. 11-OXO is an inhibitor of the enzyme11b-hydroxysteroid-dehydrogenase type 1, which converts the less active cortisone into cortisol. An other designer supplement that contains 11-OXO is 3-AD.
Steroid 64 has a characteristic, but in steroids seldom met, thioepoxide attached to ring A. It is marketed as Havoc and Hemaguno. Steroid 64 is the 17a-methyl-analog of an androgen that has been developed by Japanese chemists as a drug against breast cancer.
Steroids 65 and 66 are THP-ethers of steroids with a heterocyclic aromatic ring attached to ring A. Steroid 65 is marketed as Prostanozol and Orastan-E. Doping hunters have detected it sometimes in the urine of weight lifters and long distance runners. Steroid 65 resembles the classical anabolic steroid Stanozolol, but it lacks the 17-methyl group of the latter, and the hydroxyl group is protected as its THP-ether. This THP-ether will probably hydrolyze already in the acidic environment of the stomach.
Steroid 66 is the active ingredient in designer supplements like Furazadrol and Furaguno. It is developed in the sixties by the Japanese company Daiichi. It resembles the Japanese anabolic Furazabol, but also in this case there is no 17-methyl group and the hydroxyl group is again protected as a THP-ether
Steroids 67, 68 and 69 all have a 6a-methyl group. The last two are analogs of progesterone. Steroid 67 is an inhibitor of the enzyme aromatase. Together with steroid 68 it is an active ingredient in the designer supplement Methyl 1-P, at least according to the producers. Steroid 69 is, again according to the producer, a component in meal replacement supplements and in sports drinks.
|
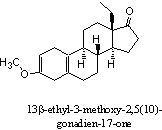
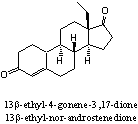
Addition: X-Mass
The steroid in the figure is 13b-ethyl-3-methoxy-2,5
ethyl-nor-androstenedione. The steroid in X-mass is quickly converted into 13b- ethyl-nor-androstenedione in the acidic environment of the stomach. This androgenic metabolite is depicted in the figure. Steroid 13b-ethyl- nor-androstenedione shows some resemblance with the designer steroid Norbolethone.
|
Conclusion
All the steroids in this chapter have been found in preparations, termed as food supplements by the producers or the dealers. These supplements can be found in supplement shops and in internet shops. In the advertising for food supplements on internet, usually the term “anabolic steroid” is avoided carefully. Mostly more descriptive indications are used, and among these the name “prohormone” is popular.
Many steroids in food supplements are indeed prohormones. However, it is disputable whether there is an actual difference between prohormones and real anabolics, when it is the intention, and often indeed the case, that enzymes will convert the prohormone in the body into the real anabolic. Several steroids in food supplements are full blown anabolic steroids themselves
We have mentioned in this chapter 74 different steroids, but the number of products in which they can be found is much larger. Years ago the American Consumer Labs. com, a company which analyses food supplements, estimated the number of hormonal supplements at more then eighteen thousand. [63]
The legal status of these designer supplements is complex. In The Netherlands and in Belgium they are considered as medicines and free trade is forbidden. Nevertheless, designer supplements circulate also in these countries. Users buy them on internet and some supplements with steroids are simply for sale in Dutch and Belgian shops.
The American law forbids some of these products, but others are for sale. Sports organizations consider them as doping, but not all the steroids mentioned above are put on their lists (see chapter 21). Besides it may be possible that doping hunters can not trace down all these steroids in their doping analyses.
We emphasize again that these steroids are present in food supplements in pharmacological doses. We are not talking about the hormonal pollution of food supplements, which sometimes have led to positive doping tests of sportsmen. These polluted food supplements probably have no effect on performance or health. The steroids in the food supplements mentioned above, certainly do show such effects.
This situation is a matter of concern. A category of food supplements is on the market which contain forbidden and potentially dangerous steroids. These steroids mostly have not been tested on human. Very little or nothing is known about possible side effects. In addition these food supplements are marketed in such a way that consumers will or can not always know what they are using. Finally, it regularly occurs that these food supplements do not really contain the active ingredients that are mentioned on the label. [64]
Look before you leap.
[1] Labrie F.; Luu-The V.; Belanger A.; Lin S.-X en Labrie C. J. Endocrinologie (2005) 187, 169-196.
[2] Labrie F.; Luu-The V.; Martel C.; Chernomoretz A. Calvo E.; Morissette J. en Labrie C. J. of Steroid
Biochemistry and Molecular Biology (2006) 100, 52-58.
[3] Earnest C.P.; Olson M.A.; Broeder C.E.; Breuel K.F.; en Beckham S.F. European Journal of Applied Physiology
(2000) 81, 229-232.
[4] Arnold P. US patent 5880117.
[5] Uralets V.A. en Gilette P.A. Journal of Analytical Toxicology (1999) 25, 357-366.
[6] Van Gammerden D.; Falk D. en Antonio J. Nutrition (2002) 18, 734-737.
[7] Page S.T.; Marck B.T.; Tolliver J.M. en Matsumoto A.M. Endocrinology. 2007 Dec 20 [Epub ahead of print].
[8] Frye C.A. Pharmacology, Biochemistry and Behavior (2007) 86, 354-367.
[9] Ergogenics, 10/12/2006.
[10] Schänzer W. en Donike M. Analytica Chemica Acta (1993) 275, 23-48.
[11] Nutting E.F.; Klimstra P.D. en Counsel R.E. Endocrinologica (1966) 53, 635-643.
[12] Nutting E.F.; Klimstra P.D. en Counsel R.E. Endocrinologica (1966) 53, 627-634.
[13] Abell L.L. en Mosbach E.H. Journal of Lipid Research (1968) 9, 98-102.
[14] Ringold H.J.; Batres E.; Halpern O. en Necoechea E (Syntex) Journal of the American Chemical Society
(1959) 81, 427-432.
[14a] Shah N.L.; Zacharias I.; Khettry U.; Afdhal N. en Gordon F.D. Clin Gastroenterol Hepatol. 2008 Feb;6(2):255-8; Jasiurkowski B.; Raj J.; Wisinger D.; Carlson R.; Zou L.; en Nadir A. Am J Gastroenterol. 2006 Nov;101(11):2659-62; Kafrouni M.I.; Anders R.A. en Verma S. Clin Gastroenterol Hepatol. 2007 Jul;5(7):809-12.
[14b] Llewellyn W.C. US patent 6242436.
[15] Friedel A.; Geyer H.; Kamber M.; Laudenbach-Lechowsky U.; Schänzer W.; Thevis M.; Vollmer G.; Zierau O. en Diel P.
Toxicology Letters (2006) 165, 149-155.
[16] Arnold P. US patent 2001056087.
[17] Arnold P. US patent 2001041698.
[18] Neumann F.; Wiechert R. (Schering-Berlin) Arzneimittel-Forschung (1965) 15, 1168-1184.
[19] Pelc B. Tsjechisch patent nr CS 110079.
[20] Counsel R.E.; Klimstra P.D. en Cotton F.B. (Searle) Journal of Organic Chemistry (1962) 27, 248-253.
[21] CIBA Belgisch patent BE 638956.
[22] Llewellyn W.C. Us patent 2003027805.
[23] Galletti F. en Gardi R. Steroids (1971) 18, 39-50.
[24] Foell T.J.; Rees R.W.; Bright R.E. en Smith H. Wyeth labs. Chemistry and Industry (1967) 34, 1452-1453.
[25] Ergogenics 15/4/2006.
[26] Duerbeck H.W.; en Bueker I. Biomedical Mass Spectrometry (1980) 7, 437-445.
[27] Lala A.K. en Kulkarni A.B. Steroids (1973) 22, 763-766.
[27a] Ojasoo T.; Delettre J.; Mornon JP.; Turpin-VanDycke C. en Raynaud J.P.; J Steroid Biochem. (1987) 27, 255-269.
[28] Perelman M.; Farkas B.; Fornefeld E.J.; Kraay R.J. en Rapala R.T. Journal of the American Chemical Society
(1960) 82, 2402-2403.
[29] Nutting E.F. en Calhoun D.W. Searle Endocrinology (1969) 84, 441-442.
[30] Koert W. en De Groot Ae. Sport en Fitness (2007) 25, 141 68-70;
Ergogenics 19/8/2007.
[31] Covey D.F. en Hood W.F. Cancer Research (1982) 42, 3327-3333.
[32] Schwarzel W.C.; Kruggel W.C. en Brodie H.J. Endocrinology (1973) 92, 666-680.
[33] Numazawa M. en Yoshimura A. Journal of steroid biochemistry and Molecular Biology (1999) 70, 189-196.
[34] Numazawa M.; Oshibe M. en Yamaguchi S. Steroids (1997) 62, 595-602.
[35] Breuggemeier R.W.; Li P.K.; Chen H.H.; Moh P.P. en Katlic N.E. Journal of Steroid Biochemistry and Molecular
Biology (1990) 37, 379-385.
[36] Brodie A.M. Cancer Research (1982) 42, 3312-3314.
[37] Kneller B.W. US patent 2006154909.
[38] Elbrecht A.; Yang Y.T. en Smith R.G. European patent 497570.
[39] Marsh D.A.; Brodie H.J.; Garrett W.; Tsai-Morris C,H, en Brodie A.M.H. Journal of Medical Chemistry
(1985) 28, 788-795.
[40] Rowlands M.G.; Foster A.B.; Mann J.; Pietrzak B.; Wilkinson J. en Coombes Steroids (1987) 49, 371-382.
[41] Sala G. Hormonal Steroids (1964) 1, 67-75.
[42] Kohler M.; Parr M.K.; Opfermann G.; Thevis M.; Schlörer N.; Marner F-J. en Schänzer W. Steroids (2007)
72 278-286.
[43] Llewellyn W.C. US patent 2003229063.
[44] Abraham S. US patent 2003199487.
[45] Covey D.F. en Hood W.F. Molecular Pharmacology (1982) 21, 173-180.
[46] Camerino B.; Patelli B. en Seiaky R. It patent 19610210, US patent 3068247.
[47] Abraham S. US patent 2003199466, US patent 2004002483.
[48] Numazawa M.; Mutsumi A. en Tachibana M. Biochemical Pharmacology (1996) 52, 1253-1259.
[49] Numazawa M. Sugiyama T. en Nagaoka M. Biological and Pharmaceutical Bulletin (1998) 21, 289-292.
[50] Arnold P. Us patent 2005059646.
[51] Rohle D.; Wilborn C.; Taylor L.; Mulligan C.; Kreider R. en Willoughby D.
J Int Soc Sports Nutr. 2007 Oct 19;4:13.
[52] Willoughby D.S.; Wilborn C.; Taylor L. en Campbell W. Int J Sport Nutr Exerc Metab. 2007 Feb;17(1):92-108.
[53] Numazawa M.; Shelangouski M. en Nagasaka M. Steroids (2000) 65, 871-882.
[54] Lardy H.; Marwah A. en Marwah P. Vitamins and Hormones (2005) 71, 263-299.
[55] Romero T. US patent 2006233828.
[56] Zweifel J. US patent.
[57] Zenk J.L. US patent 2004062606.
[58] Loria R.M.; Padgett D.A. en Huyth P.N. Journal of Endocrinology (1996) 150.
[59] Christensen B.G. en Webb T.R. WO 9610527.
[60] Schumann W. Die Pharmacie (1991) 46, 850-654.
[61] Ergogenics 20/4/2006.
[62] Ergogenics 3/4/2006.
[63] New York Daily News 25/12/2005.
[64] Musshof F.;Daldrup T. en Ritsch M. Journal of Forensic Sciences (1997) 42, 1119-1125.