Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 15/9/2007
18. Designer Steroids
Aede de Groot, Willem Koert
The Balco affair in 2003 has brought the discussion about designer steroids to the attention of the general public. However, most people hardly know what designer steroids really are, and probably the same is true for reporters and many chemists.
The word design does not induce thoughts about chemists designing new compounds for special tasks. Nevertheless the design of new molecules for medical applications has become general practice. That process is based upon profound scientific knowledge about interactions between molecules in the body, such as the interaction between anabolic steroids and the androgen receptor.
The development of new orally available and more and more selective anabolic steroids has in fact been, and still is, one continuous design process. In the beginning the design was little more than random modification of existing structures followed by testing, but gradually the design of new anabolic steroids has become more effective. Nowadays this has culminated in the design of non-steroidal anabolic molecules, the so called Selective Androgen Receptor Modulators (SARM's), and recently several promising results have been published. [1]
In the Chapters 12 en 13 we already have mentioned some designed steroids without pinpointing them as such. In those chapters we have called them chemical tricks to prevent metabolic transformations or to optimize their anabolic effects.
The present meaning of the indication designer steroid has a more vulgar origin. The name has been introduced for effective anabolic steroids which could or can not be detected in routine doping tests. Athletes can use these anabolics without punishment and thus fool the competition. Also the designers are often not as original as they should be. They find their designer anabolics in old literature or in patents of pharmaceutical companies. They select an unknown anabolic steroid that never has appeared on the market, change or adapt it a bit and try it out. They do use the existing knowledge and possibilities in a clever way.
In this chapter we first discuss why doping hunters have problems with the detection of new designer steroids. Next we explain how illegal producers may find new effective anabolic steroids and we will illustrate this with a few examples. Finally a few ideas about new designer steroids are presented.
How do the usual doping tests work?
The mass spectrometer is the most important instrument for detection of anabolic steroids in blood or urine. It is the best instrument for the screening of large amounts of samples. The mass spectrometer weighs the molecule, breaks it in pieces and detects in which pieces it has fallen apart. An analytical chemist can reconstruct the structure of the original molecule from these pieces.
Doping hunters first weigh the molecules in their samples. They do that by looking at the molecular ion peak, usually called the mol peak. This mol peak corresponds with the molecular weight of the steroid. The molecular weight is the sum of the weights of all atoms in the molecule (the atomic weight of H=1, C=12 and O=16). When this molecular weight changes because some groups are added or left out of the molecule, the mol peak also appears on a different place in the mass spectrum.
The mass spectrometer screens for instance samples for the presence of methyltrenbolone. This steroid has two methyl groups at C13 and C17. In the designer steroid norboletone two ethyl groups are present on these places (see figure 2).
The bruto formula of methyltrenbolone is C19H24O2, and its molecular weight is 284. The bruto formula of norboletone is C21H32O2 and its molecular weight is 316. In a sample that does not contain methyltrenbolone but does not contain norboletone, there will not appear a mol peak of methyltrenbolone at 284. The mass spectrometer then does not look further because he is not programmed to do so when large amounts of samples have to be screened. Thus norboletone with a mol peak at 316 is not detected. In the meantime doping hunters also screen samples for norboletone, but in former times when this was not the case and it was not detected in standard doping tests.
There are thousands of steroids known. When one does not know for which one to look, and that is the case with unknown designer steroids, it is like looking for a needle in a haystack.
A more accurate method in mass spectrometry is presented by the fragmentation pattern of a molecule. Screening of this pattern is necessary to determine the structure of a steroid unambiguously. When, for instance a mol peak does appear in the mass spectrum at 284, where one would expect one for methyltrenbolone, the spectrometer looks further. The peak pattern of methyltrenbolone is characteristic for that compound. The mass spectrometer can distinguish it from other compounds that have the same mol peak at 284 and that may be present in the sample as well. Unknown steroids also give unknown peak patterns and are not recognized by the mass spectrometer. In this way norboletone escaped detection for a long time, besides it was not on the list of forbidden compounds.
In principle doping hunters can detect all kinds of steroids in blood or urine samples. Especially when a sample of the new steroid is available, the problem usually can be solved. It does take some effort but with the help of bio assays, advanced mass spectrometry and NMR they will manage. These investigations are expensive and time consuming and not feasible for the hundreds of samples, which are taken during important matches. These samples are treated in a standard way and only screened for known forbidden compounds, which are on the lists of sports organizations, no less and no more.
How are new effective designer steroids discovered?
Not so many designer steroids are known. That is not strange because designer steroids are designed to stay unknown. There are however ample possibilities to design new steroids, as will be shown further on. The largest restriction is probably the availability of suitable starting materials for their synthesis. When inexperienced people have to carry out such a synthesis in illegal and often primitive laboratories or in small ill equipped chemical factories, it should not be too difficult. It must be possible to synthesize the new designer steroid in one or two simple chemical reaction steps, starting from a steroid that is for sale on the market.
Designer steroids also should be effective. Especially top sportsmen, or those who want to become one, are inclined to use these steroids. They want to see results, and that may be a problem. Producers of new designer steroids do not have possibilities to test their products extensively on humans. Such a research and testing program immediately will bring everything in public and that is just what they want to avoid.
However, in the fifties and sixties of the former century, the top years of steroid chemistry, thousands of steroids have been synthesized and tested, mainly by pharmaceutical companies but also in university laboratories. That research aimed at the development of new medicines for muscle wasting, post operational treatment, anaemia, menopause problems, hormone related cancers and hypogonadism. Sportsmen and producers of food supplements and designer steroids soon understood that these steroids also showed very interesting properties for their purposes. Much of that research has been published in patents and in scientific papers. Further on we show in a few examples how producers use this information to find or design new desiner steroids.
Many, but not all, of these steroids have been tested for their anabolic/androgenic activities, but often only in animals, mostly in rats. It is then known that they have or have not anabolic activity, but side effects in humans are usually not detected. The rat is not a suitable test animal to discover that kind of effects. Tests on humans are expensive and, also in former times, such tests have been performed only for a limited number of promising steroids. The side effects of steroids only become visible after testing in humans. Not many of these test results have been published. Side effects are also seldom mentioned in patents.
The reason that some active anabolic steroids never have appeared on the market is often only known by the pharmaceutical companies that have made and tested them. Too many side effects has often been an important reason. The producers of designer steroids do not bother too much about that. The users apparently have the same attitude problem, or they simply choose to deny it.
As illustration, we now will tell something about the history of the three designer steroids that are depicted in Figure 1. Nowadays doping hunters can detect these steroids in routine tests and therefore they do not anymore fulfill the preconditions for a designer steroid. These designer steroids have been knocked from their pedestal.
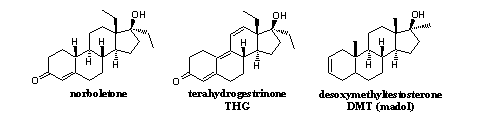
Figure 1
Norboletone
Norboletone does not meet all the conditions for a good designer steroid because it was a known compound. The anabolic steroid has been synthesized and tested by the American pharmaceutical company Wyeth. [2] The testing has been carried out by the disputed Leonard LeVann, the medical director of the Canadian institute for mentally deficient children Red Deer. [3] LeVann tested norboletone on the children from his institute, and according to his reports, norboletone was a mild anabolic compound. Later on, during the instituted legal proceedings of the children against LeVann, it appeared however that the side effects were not mentioned in his reports and that he had not worked very accurately. [4]
Despite the positive reports of LeVann, norboletone has never been put on the market by Wyeth. Several studies about the biological effects of norboletone have been published in the scientific literature in the second half of the sixties. [5-8]
When chemist and supplement designer Patrick Arnold red about these studies in the nineties of the former century, he immediately became enthusiastic. "On paper it is a real winner", he wrote in 1995, posting in the newsgroup misc.fitness.weights. "It is a strong anabolic and not very androgenic steroid."
After his decision to bring norboletone on the market as a designer steroid, he had to find a way to synthesize and produce the compound. A general multi step method for the synthesis of this kind of steroids is known in the literature and is shown in Chapter 15, scheme 6.
However, norboletone also can be synthesized in only one step by reduction of norgestrel, a steroid that has been developed for contra conception pils, and that is for sale on the market (see Scheme 1). A problem in this synthesis is that the triple bond at ring D must be reduced without reducing the double bond in ring A. Chemists know that there is a difference in sensibility for reduction between these two multiple bonds. With the right catalyst, a selective reduction of the triple bond can be achieved easily.
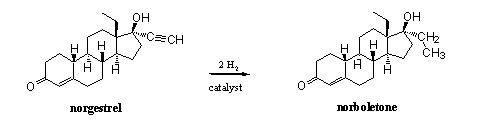
Scheme 1
The designer steroid norboletone became known as 'The Clear' because at that time it was not detected in routine doping tests. Doping hunters did not look for it because they did not know that it was used and it was not on the lists of forbidden compounds. It was only in 2002 that the cyclist Tammy Thomas was caught on the use of norboletone. [9] When reporters questioned her coach Pat McDonough, he told them that it had struck him that in the last years a striking transformation of her body had occurred. "The upper part of her body has grown, she has got a lower voice and hairs have appeared on her face", McDonough said. Apparently norboletone also showed androgenic activities.
Not many sportsmen have been caught on the use of norboletone, because there was a leak in the organisation of the doping hunters. Somebody from their laboratory warned that a test for norboletone was under development. [10] In an E-mail campaign coaches and sportmen were warned to get rid of the bottles with Arnolds anabolic. However, the "Clearman", as Patrick Arnold was nicknamed, already had a new product in the pipeline, and that was THG.
Tetrahydrogestrinon (THG)
THG was a real designer steroid. It was a new unknown compound. Several steroids that resemble THG rather closely are mentioned in a patent from 1969 of the French pharmaceutical company Roussel-UCLAF. [11] In this patent the 13b-ethyl-17a-methyl (see Figure 2) and the 17a-ethyl-13b-methyl analogs of THG are mentioned but nothing is published about the 13b,17b-diethyl-compound, THG itself.
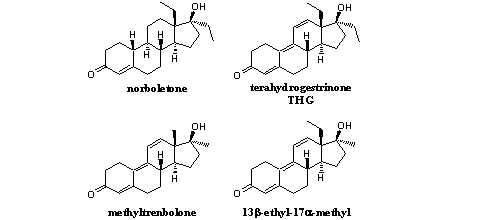
Figure 2
The high anabolic activity of the 13b-ethyl-17a-methyl compound (see Figure 2) is clearly mentioned in the patent. From tests of other series of anabolic steroids may be concluded that 17a-ethyl analogs usually are a bit less active then the corresponding 17a-methyl analogs, but there is not a big difference.
A third indication for the anabolic activity of THG may be concluded from the high activities of norboletone and (methyl)trenbolone (see Figure 2). The chemical structure of THG is a combination of the A-, B-, and C-rings of trenbolone and the D-ring of norboletone. The designers of THG have assumed that THG probably should have anabolic activity also and later on it was shown that that is indeed the case.
THG is not a simple steroid and it takes several steps to synthesize it. Arnold solved this problem in a similar way as with norboletone, by selective reduction of gestrinone (see Scheme 2). Gestrinone is the active compound in some gynaecological preparations and it is produced and marketed by several chemical companies.
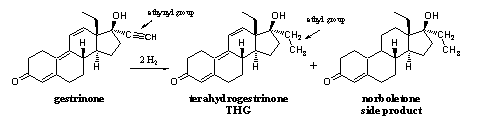
Scheme 2
Starting from gestrinone, chemists can synthesize tetrahydrogestrinone (THG) in one reaction step. The triple bond (the ethynyl group) is reduced catalytically with two molecules of hydrogen (2 H2). [12-14] In this way four hydrogen atoms (tetrahydro) are attached to the ethynyl group, which in this way is converted into an ethyl group. In this way gestrinone changes into terahydrogestrinone or THG (see Scheme 2).
The reduction of the ethynyl group for the synthesis of THG is a bit more tricky then in the case of norboletone, because also the double bonds in the B- and C-ring are sensitive for reduction. Chemists first had to investigate how to perform the reduction in a selective way. The producers have not been fully successful in that, because in the sample in which THG was detected for the first time, also some norboletone was present. Norboletone is the product that is formed when also these double bonds in the B- and C-ring are reduced. [12]
In Europe the use of THG has been restricted to track and field sport, but in the USA it was used also in football and baseball. According to the users it is a powerful compound, maybe even too powerful for athletes. "I retained water", said sprinter Tim Montgomery in a hearing. "My muscles seemed to explode. I became bigger and stronger, but not faster. It is a drug for bodybuilders, not for sprinters." [13]
Doping hunters did not detect THG. They did not know the compound and besides it behaves in an abnormal way during routine steroid analyses. In these tests first a trimethylsilyl derivative is prepared from the steroids in the sample. The trimethylsilyl derivative of THG is not stable at higher temperature and decomposes in the gaschromatograph during the analysis. [14]
Doping hunters got a small quantity of THG, in a used syringe, played into their hands by a coach. In this sample sufficient material was present to elucidate the structure of THG. [14] Once this structure was known, the analytical problem could be solved quickly. Chemists synthesized more THG and this was made at the disposal of doping hunter to develop tests. [15-17]
Also research about the anabolic activity and the possible side effects of THG has been carried out immediately. [18-21] This has been done out of curiosity, but also to enable authorities to put THG on the lists of forbidden compounds. For that it is necessary to shown that the compound indeed has anabolic activity. In all these investigations the anabolic activity of THG is confirmed, although not all scientists come to the same conclusion about the degree of activity. THG also shows androgenic activity and binds to the progesterone-, glucocorticoid- and mineralcorticoid-receptors. There are indications that THG is liver toxic. [21]
In summary we can say that THG is a role model for a designer steroid. It was an unknown compound, but in the literature knowledge about similar steroids with anabolic activity was available. It was not detected by routine doping tests. A suitable starting for its synthesis in only one step was available on the market and that made THG a commercially interesting product.
Desoxymethyltestosterone (DMT, madol)
During the police investigations of Balco, it became clear that the company had another invisible anabolic steroid on stock. In this case it also was a steroid that was known in the literature, just as with norboletone. Desoxymethyltestosterone is first mentioned in a patent from 1961. [22]
The scientific name of the compound is 17a-methyl-5a-androst-2-een-17b-ol, but doping hunters usually call it "madol". Other names are desoxymethyltestosterone or DMT. According the patent, the anabolic activity of madol is comparable with that of methyltestosterone. In a publication from 1961 investigators found after oral administration of madol to rats, an anabolic activity that is two to five times that of methyl testosterone. The androgenic activity is 0.4 to 0.6 that of methyltestosterone. [23]
From a chemical point of view, madol is rather different from norboletone and THG. There are no ethyl groups present on C13 and C17 in the structure of madol, but methyl groups. The most significant difference is found in ring A of madol. There is no carbonyl group at C3 and also the D4-double bond is absent. Instead there is a D2-double bond in ring A.
Scientists were surprised about the good anabolic and low androgenic activity of madol. Until then they assumed that a carbonyl group at C3 was essential for a good interaction with the androgen receptor and consequently for good anabolic activity. This apparently is not necessarily so.
For that reason much research has been carried out on madol and on other steroids without a C3 carbonyl group. [24-31] In these studies the good anabolic and low androgenic activity of madol is confirmed. Recently the Centre of Preventive Doping Research of the German Sports University in Colone has investigated the properties of madol again. [32] In these investigations it is concluded that madol binds selectively to the androgen receptor and that it has no affinity for the other steroid receptors. Madol has a strong anabolic and low androgenic activity and shows SARM like properties. [1] [32] The investigators also found an enlargement of the heart muscle, a side effect they can not yet explain and that may lead to some concern.
The doping hunters of WADA developed a test for this designer anabolic and reinvestigated old urine samples of elite athletes. All these tests were negative. Apparently Balco has not brought this steroid on the market. "This puts us ahead of the dopers", said WADA official Oliver Rabin triumphantly in Februari 2005 in an interview. [33] However, on 18 October the Washington Post published an article that gave the lie to his words and shook the supplement industry to its roots. [34]
In that article the famous doping hunter Don Catlin reported that his group had found designer anabolics in seven food supplements that were sold in webshops. One of these was madol, the designer steroid that never had been put on the market according to WADA. Thousands of sportsmen, especially bodybuilders, had used the designer steroids.
DMT is the active principle in supplements as 'Phera-Plex' and 'Ergomax LMG'. The producers describe the steroid on the label as als 17a-methyl-etioallocholan-2-en-17b-ol or 17-methyl-D2-etioallocholane. These are old fashioned names (see Chapter 5), probably used by the producers to put the authorities on the wrong foot.
Users apparently do known the active compounds in Phera-Plex and Ergomax. In internet blogs they tell about their gain in lean muscle and force, which is more then may be expected from a common food supplement.
When, after appearance of the Washington Post article, WADA official Rabin was asked again about his opinion, he was not amused. "It's not very difficult for some smart chemist to bypass the law", he remarked. [34] With respect to anabolic steroids, United State judges follow the list of forbidden compounds. What is on the list is not allowed, what is not on the list can be used. A small change in the chemical structure of a steroid that is on the list, changes it in something that can be put into a food supplement.
Possible new designer anabolics
The design of new steroids is relatively simple. In Chapter 15 we have shown how to do this for prohormones. A similar process can be used to speculate about new designer steroids as is shown below.
New designer steroids may have for instance:
- an extra methyl group, mostly on C7, C1, C2 and of course on C17, but possibly also on C4 and C6.
- an extra ethyl group, mostly on C13 and C17.
- more extra methyl or ethyl groups on combinations of the positions mentioned above.
- no methyl group on C19, possibly in combination with extra methyl or ethyl groups elsewhere in the molecule.
These are speculations about addition or omission of extra methyl or ethyl groups but other possibilities are:
- the introduction of one or more extra double bonds.
- the introduction of an extra hydroxyl or carbonyl group.
- the introduction of an extra F or Cl atom.
These are familiar variations, which all have been tried by chemists in the past, but not on every known steroid. There are of course many other substituents that can be introduced on basically all places in the steroid, but we will not go into that. Experience has learned that many variations in the steroid are tolerated with preservation of most of its activity, but big changes are not allowed.
It is not clever to introduce variations in the structure of a new designer steroid, that ban be converted by enzymes in the body in some known anabolic steroid. This immediately leads to positive doping tests. The designer steroid is then just a prohormone. In Chapter 15 we have already mentioned such conversions and also the same warnings are given there. New designer steroids must be designed in such a way that also their metabolites are unknown steroids.
Analogs of madol
Madol is a suitable steroid to illustrate the possibilities for the development of new designer steroids. We restrict ourselves here to variations with a 13b-methyl- or –ethyl group and/or a 17a-methyl- or –ethyl group, with or without a 19-methyl group. Then eight variations of madol are possible, which are shown in Figure 3. A literature search reveals that four of these analogs are known, but also that the four framed 17a-ethyl-madol-analogs are unknown new compounds. Maybe they have been synthesized by some pharmaceutical company, but they have - as far as we know - never been published.
Next to madol, also other 17a-alkyl-substituted 2-androstene-17b-ol steroids, among them the 17a-ethyl-steroid, are mentioned in a patent of Huffman. [22] Also the 19-nor analogs of madol [29] [31] and some other ring A modified steroids [27] have been synthesized. The 19-nor- and the 2-methyl-analogs show, just as madol itself, good anabolic and little androgenic activity.
A reason for the obscurity of the four 13-ethyl analogs of madol may be that in the past, scientists supposed that they might show too many side effects. Another reason might have been the absence of a suitable starting steroid for their synthesis. In the meantime some suitable steroid skeletons with a 13b-ethyl group are available on the market.
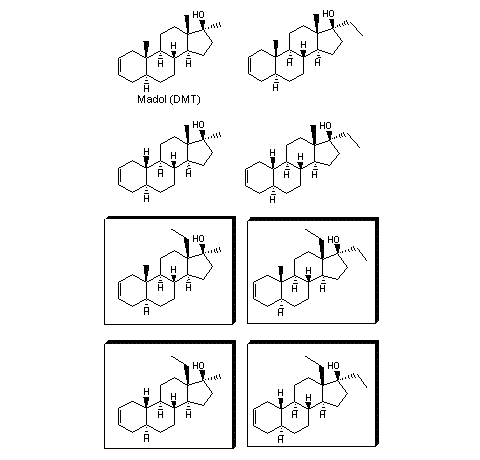
Figure 3
Since the four 13-ethyl analogs of madol are unknown compounds, all assertions that are mentioned below are pure speculation.
The framed steroids will not be detected in routine doping tests. The compounds are new and unknown and mass spectrometers will not recognize them. Perhaps an inquisitive analytical chemist will look every now and then for mol peaks that appear 14 or 28 mass units higher in the mass spectrum, which may be caused by steroids with an extra methyl or ethyl group. But even then the chance on discovery is small because he will not know for which steroid to look.
New designer steroids must be effective and they should have few side effects. We can say nothing about these items because they only can be discovered after testing on humans. Animal tests may give some indications but these are not completely reliable.
The larger scale production of new steroids has to be simple. Ordering of the steroids from a chemical company that is specialized in steroids is also a possibility. We can speculate about possible synthetic production routes, but only chemical experiments can prove that a larger scale production will be feasible for a reasonable price.
The best start for the synthesis of a new designer steroid is to buy a starting steroid that resembles the desired compound as closely as possible. For our madol analogs this means that we best can buy steroids that have already the necessary substituents on C10, C13 and C17. It will be even better when also the D4-double bond already is reduced, but with one exception, all 5aH-13b-ethyl-steroïds are unknown, and not for sale.
Therefore a possible producer will have to reduce this D4-double bond himself. After that the D2-double bond in ring A has to be constructed in a similar way as for madol itself.
We will explain this route in more detail for the 19-nor analogs of madol – the compounds with an H-atom at C10 (see Scheme 3). Other synthetic routes than the one that we will discuss here, are possible [36-39], but for the sake of simplicity we will not go into these in this book.
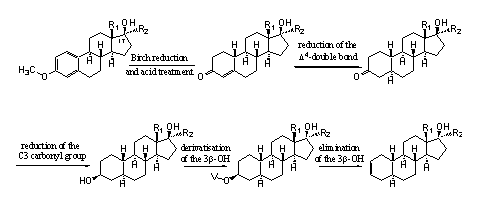
Scheme 3
In Chapter 15 we have explained already how steroids in the middle of the upper row of Scheme 3 can be prepared using the so called Birch reduction of an aromatic precursor. In Scheme 3 a general structural formula, with R1 and R2 being methyl- or ethyl groups, is shown. In Figures 4 and 5 these structures are presented in full detail. From that it becomes clear that most of these compounds are well known anabolic steroids that are available on the market.
The next step in the synthesis is the reduction of the D4-double bond for the introduction of the 5aH-atom. In the last three steps the carbonyl group at C3 has to be converted into the D2-double bond. The usual procedure for this, is first to reduce the carbonyl group to an hydroxyl group, then convert the hydroxyl group in a derivative that is easier to remove and finally to carry out this removal in a so called elimination reaction.
There are two hydroxyl groups present in the left compound on the bottom row, but the one at C17 is more sterically hindered than the one at C3. It probably will be possible to convert the last one selectively into a better leaving group. The elimination of this group can take place into two directions and next to the desired D2-double bond probably also some of the steroid with a D3-double bond will be formed. The last two steps usually are not very difficult to carry out.
It thus takes four steps to convert the known steroids from Scheme 3 into the corresponding madol analogs with a D2-double bond. This can be carried out without much trouble by a specialized chemical factory, or on a small scale, in a well equipped laboratory. It probably will be too difficult for illegal production. Somebody who likes to bring these analogs on the market better can try to find a chemical company that is willing to synthesize them.
Syntheses of designer steroïds by modification of known anabolic steroids
The general formula of the intermediate steroids in the middle of the upper row in Scheme 3 are drawn in detail in Figure 4 for the corresponding 19-nor steroids. All are known compounds and three of them have a trade name.
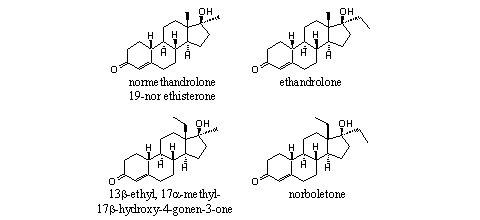
Figure 4
For the testosterone analogs the corresponding steroids are depicted in Figure 5. Also in this case these steroids are well know compounds, but they have to be prepared in a different way then the corresponding 19-nor steroids.
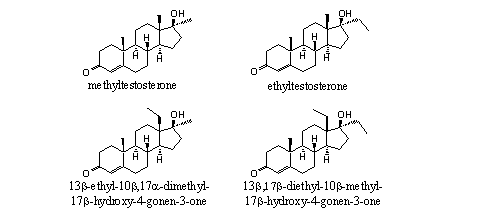
Figure 5
Most of the steroids from Figures 4 and 5 are not only active anabolic compounds themselves but they also are suitable intermediates for the synthesis of other interesting designer steroids. A method for their synthesis is shown in Scheme 4. It is relatively easy to introduce a hydroxymethylene group at C2 next to the carbonyl group at C3. We then get a whole series of analogs of oxymetholone. The oxymethylene compounds are suitable intermediates for the introduction of a methyl group at C2. In addition they are easily converted in steroids with heterocylic rings attached to ring A, as are present in stanozolol and danazol (see Scheme 4).
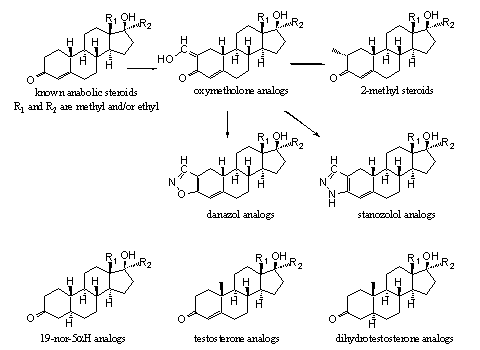
Scheme 4
The 19-nor-5a-reduced compounds left at the bottom row in Scheme 4 can be transformed in a similar way in new 5a-reduced designer steroids. The same possibilities are available for the testosterone analogs in the middle of the bottom row and for the dihydrotestosterone analogs at the right on the bottom row of Scheme 4.
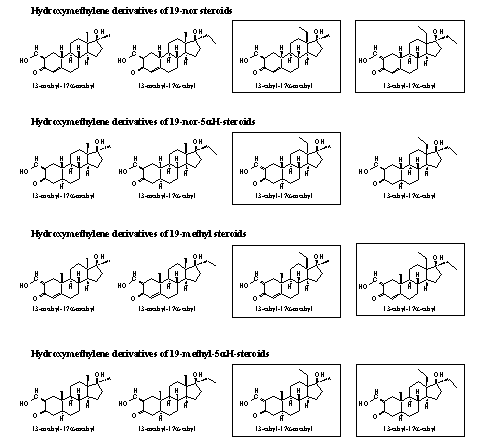
Also in Scheme 4 you can see that the hydroxymethylene compounds play a key role in the synthesis of at least three types of other possible designer steroids. For this reason we have screened the literature for the hydroxylmethylene derivatives of19-nor-steroids, 19-methyl-steroids,19-nor-5a-steroids and 19-methyl-5a-steroids. These sixteen hydroxymethylene steroids are depicted in Figure 6 and the unknown compounds are framed. [Click for larger picture]
Figure 6
It is interesting to see that scientists have extensively investigated the17a-methyl- and the 17a-ethyl-analogs of all the 13-methyl steroids (see columns 1 and 2). It is even more interesting to see that little research has been devoted to the corresponding 13-ethyl steroids (see columns 3 and 4).
We have found only two publications in which a 13 ethyl steroid is described, e.g. 2-hydroxymethylene-13b, 17a-diethyl-5aH-gonan-3-one, the second compound in the right column in Figure 6. [40] [41] Both publications are written in Chinese and in the English abstract little is mentioned about their activity.
It is also interesting that the 2-hydroxymethylene derivative of norboletone (2-hydroxymethylene-13b,17a-diethyl-4-gonen-3-one) (see Scheme 5) is an unknown compound. This compound probably can be prepared in one step from norboletone itself. When that will be successful, probably also the other steroids from Scheme 4 can be obtained without much effort.
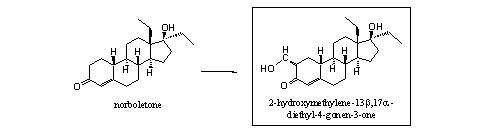
Scheme 5
The examples mentioned in Schemes 4 and 5 and in Figure 6 show the chemical possibilities in the field of designer steroids. After the Balco affair we can be sure that there are individuals who know how to exploit these possibilities.
Desiging steroids - the Pat Arnold-method
In Scheme 6 we present a last example for the synthesis of a new designer steroid. It is a variation of the method that has been used by Pat Arnold when he produced norboletone and THG. In our example we also use a compound from gynaecology as starting material: the steroid tibolone, which is prescribed for treatment of menopause complains.
The selective catalytic reduction of the triple bond at C17 can be accomplished in the same way as has been done in the synthesis of norboletone and THG. The greater steric hindrance around the double bond between the A- and B-ring probably will allow here also a selective reduction. The well known D4,9- and the D4,9,11-double bonds can be introduced next in one or two steps. The extra 7a-methyl group usually has a favorable effect on the anabolic activity. The framed steroid is not known in the literature.
In summary this compound will show probably anabolic activity, it is not known in the literature and a suitable starting material for its production is for sale. In this way the compound fulfills all conditions for a successful designer steroid.
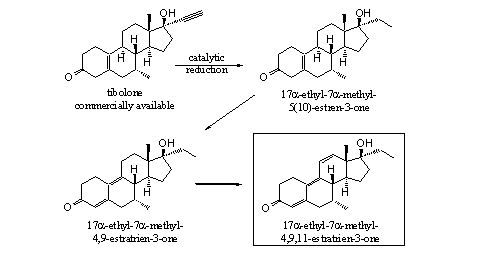
Scheme 6
Once one realizes the relative simplicity of finding new designer steroids, its understandable that doping hunters – perhaps not the managers, but certainly the technicians - entertain few illusions. The Balco affair, the discovery of norboletone and THG, and the relative easy with which Pat Arnold launched his invisible anabolics, show clearly the inadequacy of the present doping tests.
Sports writer Will Carroll, who wrote a book about the use of anabolics in American baseball, estimates that at least three illegal organizations are actively engaged in designer anabolics in the US. These- and similar organizations- have, without any doubts- already thoroughly investigated the above mentioned possibilities long ago. Maybe only the costs are prohibitive. Mostly several chemical reactions steps are required for the introduction of each extra substituent in anabolic steroids. Each step will make the end product more expensive and that will make advanced designer steroids too expensive for the common sportsmen.
On the other hand, the events around norboletone and THG demonstrate that one should not underestimate the creativity of people like Pat Arnold. When scientist and pharmacists had practically closed their chapters about anabolic steroids, he still saw possibilities. With norboletone and THG he has shown that the story about anabolic steroids is far from finished.
The examples mentioned above show that new steroid variations can be designed relatively easily. Maybe some of them are used already. It is however not so easy to find really good new anabolic steroids, with a reasonable ratio between anabolic and androgenic effects and with little side effects.
Ultimately the user is the guinea pig.
[1] Ostrovski J.; Kuhns J.E.; Lupisella J.A.; Manfredi M.C.; Beehler B.C.; Krystek S.R.; Bi Y.; Sun C.; Seethala R.; Golla B.; Sleph P.G.; Fura A.; An Y.; Kish K.F.; Sack J.S.; Mookhtiar K.A.; Grover G.J. and Hamann L.G. Endocrinology (2007) 148, 4-12.
[2] Edgren R.A. (Wyeth Labs.) Acta Endocrinologica (1963) 87, 21.
[3] Int J Clin Pharmacol 1972 Apr;6(1):54-9.
[4] National Post 28/10/1998.
[5] Edgren R.A.; Clancey P.D.; Jones R.C.; Nagra C.L.; Smith H. and Hughes G.A. Recent Progress in Hormone Research (1966) 21, 305-341.
[6] Albanese A.A. Medical Times (1968) 96, 871-881.
[7] Albanese A.A.; Lorenze E.J.; Otto L. A. and Wein E.H. Journal of Medicine (1968) 68, 2392-2406.
[8] Bertolini A. Rivista di Farmacologia e Terapia (1970) 1, 257-262.
[9] Catlin D.H.; Ahrens B.D. en Kucherova Rapid Commun. in Mass Spectrometry (2002) 16, 1273-1275.
[10] Washington Post, 8-3-2003.
[11] Vignau R.; Bucourt R.; Tessier J.;Costerousse G.; Nedelec L.; Gasc J-C.; Joly R.; Warnant J. and Goffinet B. Roussel UCLAF US patent 3,453,267.
[12] Catlin D.H.; Sekera M.H.; Ahrens B.D.; Starcevic B.; Chang Y-C. and Hatton C.K. Rapid Commun. In Mass Spectrometry (2004) 18, 1245-1249.
[13] San Francisco Chronicle, 28-6-2004.
[14] Karpiesiuk W.; Lehner A.F.; Hughes C.G. and Tobin T. Chromatographia (2004) 60, 359-363.
[15] Salvador J-P.; Sanchez-Baeza F. and Marco M-P. Analytical Chemistry (2007) 79, 3734-3740.
[16] Levesque J-F.; Templeton E.; Trimble L.; Berthelette C. and Chauret N. Analytical Chemistry (2005) 77, 3164-3172.
[17] Thevis M.; Bommerich U.; Opfermann G. and Schänzer W. Journal of Mass Spectrometry (2005) 40, 494-502.
[18] Death A.K.; McGrath K.C.; Kaslauskas R. and Handelsman D.J. Journal of Clin. Endocrinol. Metab. (2004) 89, 2498-2500.
[19] Jasuja R.; Catlin D.H.; Miller A.; Chang Y.-C.; Herbst K.L.; Starcevic B.; Artaza J.N.; Singh R.; Datta G.; Sarkissian A.; Chandsawangbhuwana C.; Baker M. and Bhasin S Endocrinology (2005) 146, 4472-4478.
[20] Labrie F.; Van L-T.; Calvo E.; Martel C.; Cloutier J.; Gauthier S.; Belleau P.; Morissette J.; Levesque M-H. and Labrie C. Journal of Endocrinology (2005) 184, 427-433.
[21] Friedel A.; Geyer H.; Kamber M.; Laudenbach-Leschowsky U.; Schänzer W.; Thevis M.; Vollmer G.; Zierau O. and Diel P. Toxicology Letters (2006) 164, 16-23.
[22] Huffmann M.N. (1961) US patent 2,996,524.
[23] Edwards J.A. and Bowers A. Chemistry and Industry (1961) 1962-63.
[24] Dorfman R.I. and Kincl F.A. Endocrinology (1963) 72, 259-266.
[25] Kincl F.A. and Pi A.F. (Syntex) Ciencia (1963) 22, 49-53.
[26] Kincl F.A. and Dorfman R.I. (Syntex) Steroids (1964) 3, 109-122.
[27] Cross A.D.; Edwards J.A.; Orr J.C.; Berkoz B.; Cervantes L.; Calzada M. and Bowers A (Syntex) Journal of Medicinal Chemistry (1963) 6, 162-166.
[28] Neumann F. and Wiechert R. (Schering) Arzneimittel Forschung (1965) 15, 1168-1184. [29] Counsell R.E.; Adelstein G.W.; Klimstra P.D. and Smith B. Journal of Medicinal Chemistry (1966) 9, 685-689.
[29] Counsell R.E.; Adelstein G.W.; Klimstra P.D. and Smith B. Journal of Medicinal Chemistry (1966) 9, 685-689.
[30] Nutting E.F.; Klimstra P.D. and Counsell R.E. (Searle) Acta Endocrinologica (1966) 53, 627-634.
[31] Bowers A.; Edwards J.A. and Orr J.C. (Syntex, 1966) United States patent 3 239 542.
[32] Diel P.; Friedel A.; Geyer H.; Kamber M.; Laudenbach-Leschowsky U.; Schänzer W.; Thevis M.; Vollmer G. and Zierau O. Toxicology Letters (2007) 169, 64-71.
[33] The Guardian, 2-2-2005.
[34] Washington Post, 18-10-2005.
[35] Described in the Anabolic Steroid Control Act of 2004.
[36] Klimstra P.D. and Counsell R.E. (Searle, 1962) United States patent 3 018 298.
[37] Counsell R.E. and Klimstra P.D. (Searle, 1965) United States patent 3 203 966.
[38] Pelc B. and Fajkos J. Czech patent 1967, CS 122950.
[39] Popisec J.; Koblicova Z. and Trojanek J. Czech patent 1968, CS 126388.
[40] Weisheng T.; Xu F. and Liao Q. Yaoxue Xuebao (1984) 19, 35-40.
[41] Li L.; Hou D.; Tao Y. and Liu Z. Zhongguo Yaoke Daxue Xuebao (1987) 18, 66-68.
|
|