Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 11/10/2007
17. Ethers and Nitrogen Derivatives
Aede de Groot, Willem Koert
Most of the steroid derivatives are esters, but also THP ethers, enol ethers, ordinary ethers, silyl ethers and nitrogen derivatives of steroids have been synthesized and marketed. Their characteristic chemical properties explain why they work in a different way and why they have become less popular then esters.
There are no enzymes in our blood, capable to hydrolyse THP-ethers, enol ethers, ordinary ethers, silyl ethers or nitrogen derivatives, as is the case with esters. Most of these derivatives are however hydrolyzed by gastric acid. When taken orally these derivatives will hydrolyze in the stomach and the active steroid is set free there. When these derivatives are injected, the active steroid mostly is set free in the lever, probably after oxidation by cytochrome P450 enzymes.
Tetrahydropyranyl-ethers (THP-ethers) and other acetals
Chemists use THP ethers for temporary protection of hydroxyl groups against undesired reactions. Later the THP group can be removed easily by acid hydrolysis. Anabolic steroids are often marketed as THP ethers following the same idea. In the body the THP group is removed to set free the active steroid.
THP ethers of steroids are only partial ethers, although the word ether is in the name. An ether can be derived from water by substituting the H-atoms by alkyl groups. In Scheme 1 first one H-atom is replaced by an ethyl group, which leads to ethanol, the common alcohol in beer, wine and stronger alcoholic beverages. When also the second H-atom is replaced by an ethyl group, an ether is formed, in this case diethyl ether, the most common ether. In an ether both C-atoms next to the O-atom have only one bond to this O-atom.
A sixmembered ring with an O-atom and two double bonds is called a pyran. When both double bonds are reduced, each with two H-atoms, four H-atoms will enter the molecule and we get tetrahydropyran (see Scheme 1). When the second H-atom at the O-atom of ethanol is replaced by this group, we get the tetrahydropyranyl ether of ethanol. Till here everything is the same in the upper and lower row of Scheme 1. Nevertheless there are differences between the two ethers.
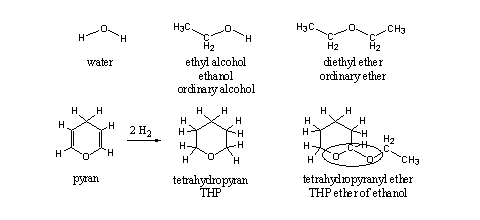
Scheme 1
On the right side of the O-atoms everything is the same, but on the left side this is not the case. In diethyl ether the left C-atom also has only one bond to the O-atom. In the tetrahydropyranyl ether this left C-atom has two bonds to two different O-atoms.
This seemingly small difference in structure means that this group has other chemical properties than an ordinary ether, and it has also a different name. This group is encircled in the structural formula and is called an acetal. Acetals are easily hydrolyzed under acidic conditions and ethers are not (see Scheme 2). The explanation for this difference in chemical behavior is known but we will not go into that in this book.
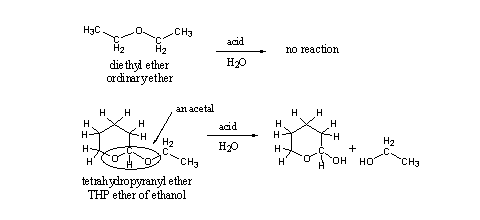
Scheme 2
The stomach contains gastric acid. Chemists know this acid better as hydrochloric acid (HCl) and this is a strong acid. Gastric acid causes a pH between 1 and 2 in the stomach and that are pretty strong acidic conditions. Chemists indicate acidity with pH values. Ordinary water has a pH of 7, which is called neutral. A pH between 0 and 7 is called acidic and a pH between 7 and 14 is called basic. In the intestinal tract the pH is slightly basic, between 7 and 8. In the blood and in muscles the pH is close to neutral.
Acetals like THP ethers hydrolyze easily under acidic conditions, which means that orally taken THP ethers of anabolic steroids will hydrolyze already in the stomach. This sets free the anabolic steroid, while it still has to pass the intestinal track and the liver. So there will be time and opportunity for metabolic transformation of the steroid to inactive metabolites.
A THP ether also can be given parentally, but the THP steroid derivative itself is not active. When the derivative does not pass the stomach, the THP ether group has to be removed in a different way. In the blood and in muscles the pH is neutral and under these conditions THP ethers are stable compounds. We also do not have enzymes in the blood, which can hydrolyze THP ethers, as is the case with esters.
However, cytochrome P450 enzymes can oxidize THP ethers in the liver. This oxidation takes place in the ring next to the O-atom. The oxidation product then decomposes spontaneously to a dialdehyde and the free steroid (see Scheme 3). This scheme is a bit speculative, because little has been published about the metabolism of THP ethers.
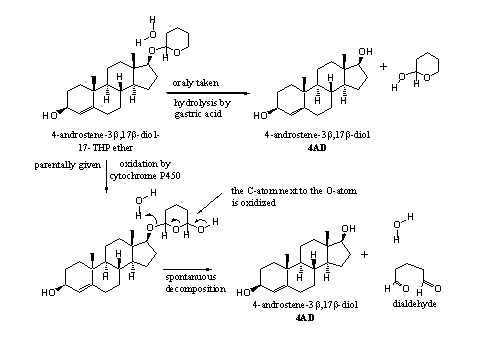
Scheme 3
The free steroid will be converted in the liver to glucuronates or sulfate esters. The free steroid also may be inactivated through bonding to Sex Hormone Binding Globuline. Probably only a small part of the steroid will reach the muscle cell.
There is a clear difference between esters and THP-ethers of anabolic steroids. Esters are given parentally and after a slow release in the blood, enzymes will hydrolyze them to the active anabolic steroid. Orally taken THP ethers will hydrolyze already in the stomach. Parentally given THP ethers are oxidized in the liver and the active steroid is set free there.
As orally taken THP ethers are already hydrolyzed in the stomach, it does not make much difference when you take the anabolic steroids itself or its THP ether. The marketing of an anabolic steroid as its THP ether probably has more to do with avoidance of patents or law regulations than with the making of better or longer lasting anabolic compounds. In Figure 1 some THP ethers of anabolic steroids are collected, which we have found in nutritional supplements.
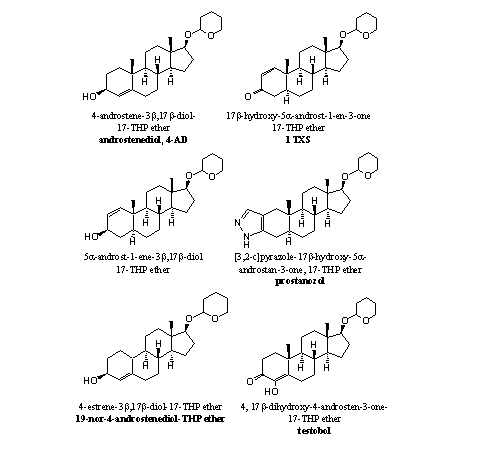
Figure 1
Acetal derivatives of steroids also may appear in different forms, as is shown in the double acetal of testosterone (see Figure 2) [1]. The C3-atom in ring A has two bonds to O-atoms and therefore it is an acetal. This group is called a dioxolan ring and it is often used by chemists to protect a carbonyl group.
The C-atom in the middle in the side chain at C17 also has two bonds to O-atoms and this methoxymethyl group is therefore also an acetal. Both acetals can be hydrolyzed with dilute acid (gastric acid). The activity of this derivative is comparable with that of testosterone when it is taken orally and it is assumed that hydrolysis in the stomach indeed takes place.
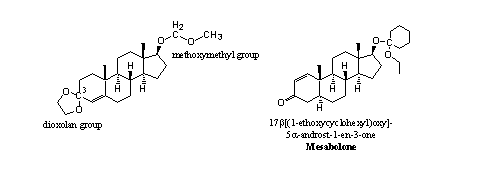
Figure 2
In mesabolone the acetal is connected to the C17-hydroxyl group. The C-atom in the cyclohexyl ring is connected to a second O-atom and therefore this derivative is also an acetal. It may be assumed that also this derivative will hydrolyze in the stomach when taken orally.
Dozens of such acetals are known as protective groups, but the THP ether and the dioxolan ring are the best known [2]. Other acetals that hydrolize at pH2, the conditions in the stomach, are mentioned in Figure 3.
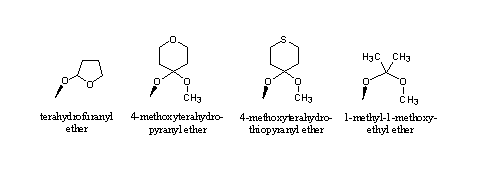
Figure 3
The tetrahydrofuranyl (THF) ether hydrolyzes a bit faster than the THP ether. The reason for this is the larger ring strain and the slightly lower steric hindrance in the five membered ring.
Also in the other structures an acetal can be recognized, they resemble the acetal in Figure 2. The situation is a bit different in each compound, which is reflected in their hydrolysis velocity. All these acetals will hydrolyze in the stomach when taken orally. Their behavior upon injection will only become clear after testing.
Enol-ethers
In chapter 14 we have already explained that in an enol the hydroxyl group is directly connected to a double bond. When next the H-atom at the hydroxyl group is replaced by an alkyl group, here an ethyl group, we get an enol ether (see Scheme 4).
The chemical behavior of enol ethers resembles that of acetals. Enol ethers hydrolyze under acidic conditions to a carbonyl compound and a hydroxyl compound. The details of this hydrolysis are known, but here we only give the end result. Both types of compounds from this hydrolysis, the hydroxyl group and the carbonyl group, can be used to set free the steroid from its enol ether derivative (see Scheme 4).
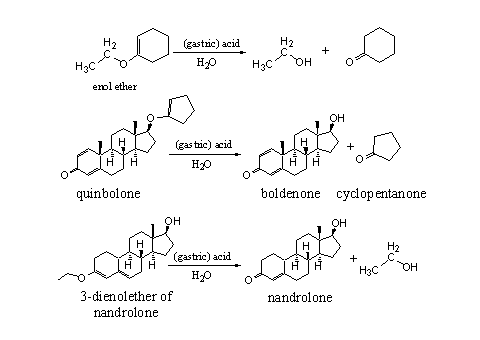
Scheme 4
Hydrolysis under the acidic conditions in the stomach of the enol ether quinbolone, sets free the C17 hydroxyl group of the anabolic steroid boldenone, together with cyclopentanone. In the second example in Scheme 4 the dienol ether of nandrolone is hydrolyzed to set free the 4-en-3-one moiety of this steroid [3].
The remarks about the metabolism of THP ethers and acetals also count for enol ethers. Enol ethers will hydrolyze already in the stomach and it is not sensible to take such a derivative orally instead of the anabolic steroid itself. There are also no enzymes known, which can hydrolyze enol ethers after injection. Just as with acetals, oxidation in the liver can take place to set free the active steroid.
In food supplements we also have found an interesting combination of a THP ether and a dienol ether (see Scheme 5). It concerns a derivative of the aromatase inhibitor 6 OXO. Also in this case it is not useful to take this compound orally instead of 6 OXO itself, because also here hydrolysis will take place in the stomach. After hydrolysis of either the THP ether or the enol ether the well know 4-en-3-one moiety will return in the molecule.
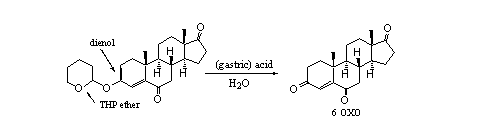
Scheme 5
Ordinary ethers
Not so many ether derivatives of steroids have appeared on the market and that is not suprising. The main goals of administration of a derivative instead of the steroid itself is oral availability, slower metabolism and elongation or modification of its activity. The derivative itself usually does not have anabolic activity.
Ordinary ethers are not very reactive compounds and we do not have enzymes in our body, which are capable to hydrolyze ethers. Ethers are also stable compounds under the acidic conditions of the stomach, this in contrast to acetals and enol ethers, which are hydrolyzed in the stomach. Oxidation in the liver probably will occur. An ether derivative of an anabolic steroid is mostly only useful when it is an active compound itself.
A reasonable number of ordinary ether derivatives of, mostly, testosterone has been investigated. It is known that 17b-methoxytestosteron has a modest anabolic activity of about 25-30% of that of testosterone itself [4]. The other ethers from Figure 3 do not have significant anabolic activity. The benzyl, allyl, propyl and 3-hydroxypropyl ethers (see Figure 3) are inhibitors of 5a-reductase [4].
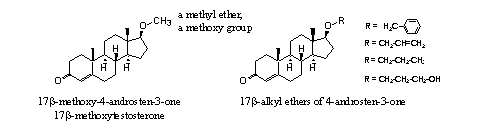
Figure 4
The methyl ether of trenbolone is marketed as the designer supplement methoxytren. After analysis of the product it proved however that no methoxy group is present in the active compound. The supplement just contains trenbolone. [5]
The pharmaceutical company Roussel-UCLAF has patented in the sixties of the former century, several ether derivatives of trenbolone. In a separate patent they claimed the methoxy ether of methyltrenbolone (see Figure 3 bottom right). This ether is more then twenty times anabolic as 17a-methyl-19-nortestosterone. [6] It is not mentioned whether this methoxy ether is toxic for the lever just as 17a-methyltrenbolone itself. The ethers shown at the bottom left in Figure 3 are orally active and exhibit anabolic and androgenic activity. [7]
From trenbolone itself, its 13b- ethyl derivative and its 7b-methyl derivative a number of ethers have been prepared which are shown in Figure 4. [8] Their considerable anabolic and androgenic activity is smaller than that of 17a-methyltrenbolone, but they have a prolonged period of action.
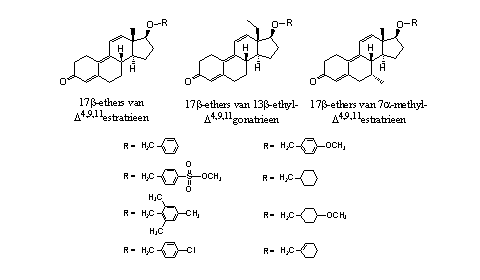
Figure 5
Silyl-ethers
We also like to mention silyl ethers here, because these ethers are used also extensively in organic chemistry as protection for hydroxyl groups. Silyl ethers are more reactive then normal ethers, due to the presence of the silicium atom (Si). Trimethylsilyl ethers hydrolyze already in water to set free the hydroxyl group. Under the acidic conditions in the stomach this goes even quicker.
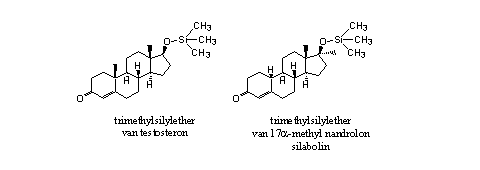
Figure 6
Injected silyl ethers have to hydrolyze under the neutral conditions in the blood or in the muscle and this is what happens with trimethylsilyl ethers. More hindered silyl ethers, with larger groups attached to the Si-atom, hydrolyze very slowly or not at all under neutral conditions. We do not have enzymes in our body, which are capable to hydrolyze silyl ethers.
The injected trimethylsilyl ether of testosterone has a larger androgenic and anabolic effect then testosterone itself [9]. The trimethylsilyl group makes the molecule more apolar. In this way it becomes better soluble in fat and is easier transported in the body. After transport to the blood a fast hydrolysis to testosterone can follow. When silyl ethers do not hydrolyze, they also do not have anabolic activity.
The anabolic activity of the trimethylsilyl ether of 17a-methyl-nandrolone (silabolin) has been investigated also (see Figure 5) [10]. The established anabolic activity is probably affected by the hydrolysis product 17a-methyl nandrolone itself.
Nitrogen derivatives
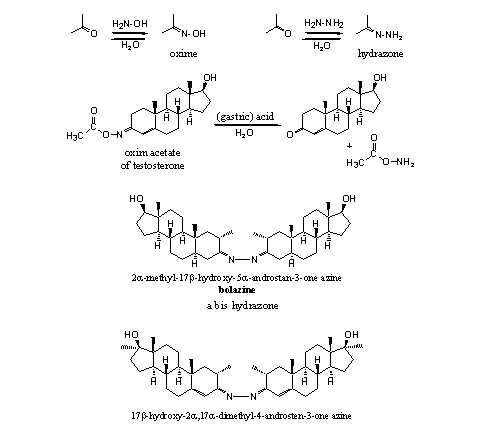
Nitrogen derivatives of carbonyl compounds are known for a long time. Chemists can synthesize these derivatives easily by reaction of a carbonyl compound with a reagent that contains an amino group. The reaction of a carbonyl compound with hydroxylamine (H2N-OH) gives an oxime, the reaction with hydrazine (H2N-NH2) leads to an hydrazone. Water is formed in this reaction. Both reactions are equilibrium reactions and a reaction with water (hydrolysis) gives back the original carbonyl compound (see Scheme 6).
Scheme 6
Several oximes and oxime esters have been patented by Searle [11] [12]. According these patents these steroid derivatives show anabolic, androgenic, esterogenic, hypocholesterolemic and antibiotic activities, but no details to substantiate these claims are given. To our knowledge, the compounds never have appeared on the market. The oxime acetate of methyltestosterone can be obtained by esterification of the hydroxyl group of the oxime.
The two azines shown in Scheme 6 have been marketed as bolazine and mebolazine. Also these nitrogen derivatives probably will hydrolyze in the stomach and the active anabolics will be set free there.
[1] Kupchan M., Casy A.F. and Swintosky J.V. Journal of Pharmaceutical Sciences (1965) 54 514-524.
[2]T.W. Greene and P.G.M. Wuts, Protective Groups in Organic Synthesis, third edition, John Wiley & sons.
[3] Ercoli A. patent GB 927165 19630529.
[4] Solo A.J., Bejba N. and May M. Journal of Medicinal Chemistry (1975) 18 165-168.
[5] W.Koert and Ae. de Groot. Sport en Fitness (2007) 25 141 68-70; Ergogenics 19/8/2007.
[6] Bucourt R. and Costerousse G. Roussel-UCLAF, French patent FR 5320 19670925.
[7] Nedelec L.; Tessier J. and Costerousse G. Roussel-UCLAF, South African patent ZA 6701974 19680417.
[8] Costerousse G. and Gase J-C. Roussel-UCLAF, US patent 3629244.
[9] Chang E. and Jain V. K. Journal of Medicinal Chemistry (1965) 9 433-435.
[10] Shishkina A.A., Ivanenko T.I., Zarubina N.A., Volzhina O.N., Angarskaya V.G., and Pivnitskii K.K. Khimiko-farmat Sevticheskii Zhurnal (1986) 20, 232-237.
[11] Mazur R.H. (Searle) US patent 3211756 19651012.
[12] Mazur R.H. (Searle) GB 996256 19650623.
|
|