Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 22/6/2007
15. Prohormones
Aede de Groot, Willem Koert
Hormones are chemical messengers. They are produced by glands and deliver their message in different parts of the body. Synthetic hormones, like anabolic steroids, are administered as drug and work in a similar way as the natural hormones (see chapter 8), at least that is the intention.
Prohormones are synthetic chemical compounds, which are transformed by enzymes in the body into natural hormones or in compounds with comparable hormonal activity. This chapter will deal with these prohormones.
When we look at natural anabolic steroids, enzymes have to convert the prohormones into testosterone. With synthetic anabolic steroids, enzymes have to convert the prohormones into the corresponding real anabolic steroid like norboletone or nandrolone.
On internet and in other literature we also have found the names prosteroids and prodrugs for compounds that are offered for sale as prohormones. Often these names are accompanied by the term "legal".
"Legal" means that the prohormone have not yet been forbidden. In Europe prohormones are forbidden. Since 2004 many prohormones are also forbidden in the United States. However on internet prohormones are easy to get and producers continue to advertise them using the usual promising terminology.
Competing sportsmen and women, who are checked regularly for doping abuse, should not use prohormones, even when they are legal. It is the intention that prohormones are converted in the body into the real anabolic steroid. This real anabolic steroid may be on the list of forbidden compounds. The prohormone, after being converted into the real anabolic, also gives all its metabolites. Doping hunters will find this real anabolic steroid and/or its metabolites in the urine or in the blood. The user is caught (as he or she should be), although he only has used a "legal" prohormone in a food supplement.
The name prosteroid is nonsense. The prohormone itself is already a steroid. Users of this name probably are focused too much on the active anabolic steroid, which is produced by enzymes from the so called prosteroid. Following this type of reasoning the term proanabolic would be better.
The term prodrug is a reasonable alternative. Chemists and pharmacists consider synthetic anabolic steroids as a medicine or drug. These compounds are developed in the first place as drug and therefore their precursors can be considered as prodrugs.
We will not use the term prodrug for precursors of synthetic anabolic steroids but we prefer the term prohormone. Although prohormones and the real anabolic steroids that originate from them, are of synthetic origine, they have to act as a hormone and that is what matters.
In this chapter we first will look at prohormones, which are converted into the natural hormones testosterone and dihydrotestosterone. After that also prohormones for synthetic anabolic steroids will be treated.
Enzymes have to convert prohormones for testosterone into testosterone itself and for that reason we can put these prohormones in or around the biosynthetic scheme for this natural anabolic steroid. This has been done in scheme 1. The framed compounds are or have been for sale on the market. It can be noticed that four of these prohormones are intermediates in the biosynthesis of testosterone, just 4-androstene-3b,17b-diol (4-AD) at the bottom of the scheme is not an intermediate in the biosynthesis. A 3b-dehydrogenase can convert this compound in testosterone and therefore it is a prohormone.
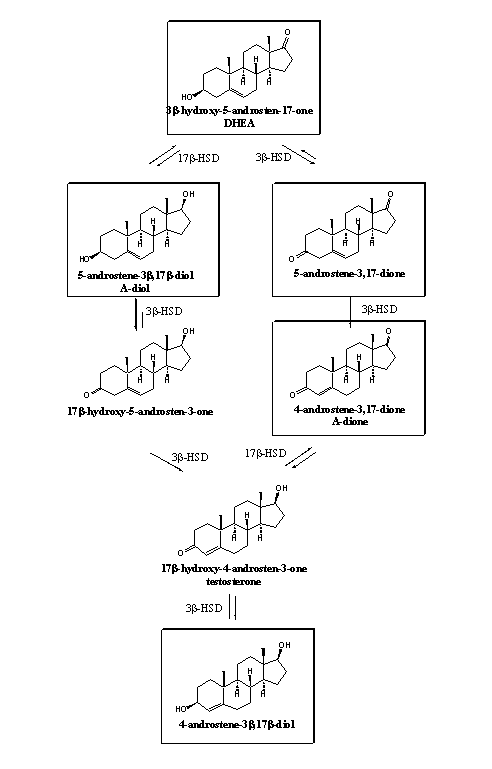
Scheme 1
All prohormones for testosterone are also prohormones for dihydrotestosterone because the enzyme 5a-reductase can achieve this conversion. The reverse reaction is not possible. Prohormones of dihydrotestostrone are not converted into testosterone. There are no enzymes known that can introduce a D4-double bond in a steroid. In prohormones that are intended only as prohormone for dihydrotestosterone the 5a-H-atom already has to be present in the prohormone. Just as in testosterone, C3 and C17 dehydrogenases can convert the prohormones in scheme 2, into dihydrotestosterone. The framed prohormones have appeared on the market.
The benefit of prohormones of dihydrotestosterone for bodybuilders and fitnessers is not clear. Dihydrotestosterone is the hormone that causes the androgenic effects and has no anabolic properties.
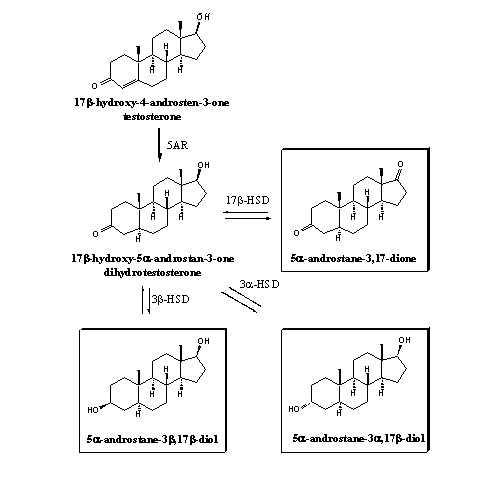
Scheme 2
It does not need much phantasy to realize that enzymes, which are capable to convert prohormones in testosterone, probably will do the same with prohormones for synthetic anabolic steroids that resemble testosterone. In the first place steroids like 17a-methyl testosterone and 19-nor testosterone (nandrolone) come in mind, but in principle there are not many restrictions.
Possibilities are:
- The introduction of an extra methyl group at C7, C1, C2, and of course at C17.
- The introduction of an ethyl group. This group sometimes is located at C13, but mostly at C17,
because of the easy synthesis of compounds with ethyl groups at this place.
- The introduction of more than one extra methyl or ethyl group on combinations of the places
mentioned above.
- The omission of the C19-methyl group, which leads to prohormones for nandrolone or other 19-nor
steroids.
- The omission of the C19-methyl group in combination with the introduction of extra methyl or ethyl
groups at other places in the molecule.
Possible enzymatic transformations for the conversion of prohormones into the real anabolic steroids are:
The conversion of a a- or b-hydroxyl group on C3 by
3a-HSD or 3b-HSD-enzymes into the desired C3 carbonyl group.
The conversion of a D5(6) double bond in combination with a
3b-hydroxyl group by
3b-HSD/D4,D5-isomerase enzymes in the desired D4-C3-carbonylverbinding.
-C3 carbonyl compound.
The conversion of a D5(6) double bond in combination with
a C3-carbonyl group by
D4,D5-isomerase-enzymes into a
D4-C3 carbonyl compound. This conversion may be achieved also by
gastric acid when the prohormone is taken orally.
The conversion of a D5(10) double bond in between the A and B ring into
a D4-C3 carbonyl compound. This possibility is only available in
19-nor steroids because only there a D5(10)-double bond is
possible. Also in this case the conversion may be achieved by gastric acid when the prohormone is taken
orally.
The conversion of a C17-carbonyl group by 17b-HSD enzymes into the desired
17b-hydroxyl group. This possibility is not available in
17a-methyl steroids because in these molecules there is no place for
a C17 carbonyl group.
In schemes 1 and 2 we can see that many of these transformations have been applied already for prohormones of testosterone and dihydrotestosterone. For analogs of testosterone, for nandrolone, for analogs of nandrolone and for many other anabolic steroids the same possibilities are in principle available.
In figure 1 five possible prohormones for 17a-methyltestosterone, depicted in the left upper corner, are collected. Methyl-4-AD has appeared on the market, but also the other steroids are known compounds. In our opinion the enzymes in the body will be capable to transform these "prohormones" into methyltestosterone itself. This is however not known for all compounds in figure 1, and sometimes it is difficult to predict their actual behavior.
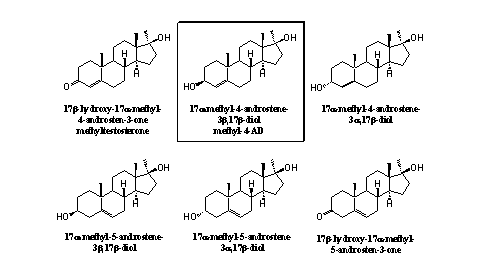
Figure 1
The extra methyl group also can be introduced at C7, C11 or C2, and it is also possible to introduce more then one methyl group, for instance at C17 and at C7, C11 or C2, or at C7 and C11, and so on. The 11a- and 11b-methyl steroids and the 11a,17b- and 11b,17a-dimethyl steroids, depicted in the right column in figure 2, are unknown compounds
For each of the compounds in figure 2 in principle the same possibilities for prohormones are available as displayed in figure 1 for methyl testosterone. Combination of the possibilities of figures 1 and 2 then gives 30 possible "legal" prohormones.
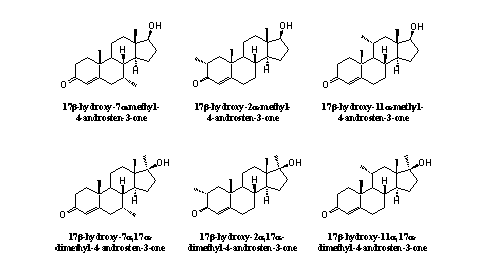
Figure 2
The methyl groups at C7, C11 and C2 in figure 2 are presented only in the a-position but they also can be put in the b-position. The same number of variants can be drawn with an ethyl group at C13 or C17, when necessary in combination with extra methyl groups. Many, but not all, of these molecules are known in the literature and in patents. Chemist can synthesize the corresponding prohormones for all of these steroids.
The anabolic steroid nandrolone (19-nor testosterone) and its analogs are the targets of metabolic conversions of 19-nor prohormones. The framed 19-nor prohormones for nandrolone in scheme 3 have appeared on the market.
Producers and researchers are not very accurate with the indication of the place of the double bond in 19-nor prohormones. The D4-double bond is indicated correctly, but in 19-nor steroids there are two possibilities for a D5-double bond. This double bond can be placed in between the A and B ring, at the D5(10)-position or in ring B at the D5(6)-position. This is often not indicated clearly [1].
The D5(10) steroids can be synthesized easily (see Scheme 4 below), also because they can be produced from steroids in anti conception pils. For that reason they are easily commercially available. When the position of the double bond is just indicated in the name as 19-nor 5-androstene, it mostly concerns the D5(10)-steroids. In androstene prohormones this problem is not relevant, because in these steroids there is no place for a D5(10)-double bond. In 5-androstenes the double bond only can be located in the D5(6)-position.
For users the exact position of the D5 double bond in 19-nor steroids is not a problem because both prohormones are converted to nandrolone. Researchers should be more accurate in their indication of the position of this double bond.
Enzymes can convert the D5(10)-steroid at the bottom of scheme 3 in the C3-carbonyl compound and next into nandrolone [2][3][4]. The carbonyl compound with the D5(10)-double bond also is a prohormone (see also Scheme 4). When taken orally it can be transformed into the desired D4-compound by gastric acid [5][6].
Enzymes can convert also the D5(6)-compounds in scheme 3 into nandrolone and the steroids on top of scheme 3 are therefore also possible prohormones. They are however more difficult to synthesize and not available in large quantities. The D5(6)-3-carbonyl compound (5(6)-estrene-3,17-dione) is not a suitable prohormone, because this compound is not very stable and it isomerizes easily to nandrolone [6]. The reason for this easy isomerisation of the D5(10)-3-one and D5(6)-3-one structures into the D4-3-one system is the greater stability of the conjugated system (C=C—C=O).
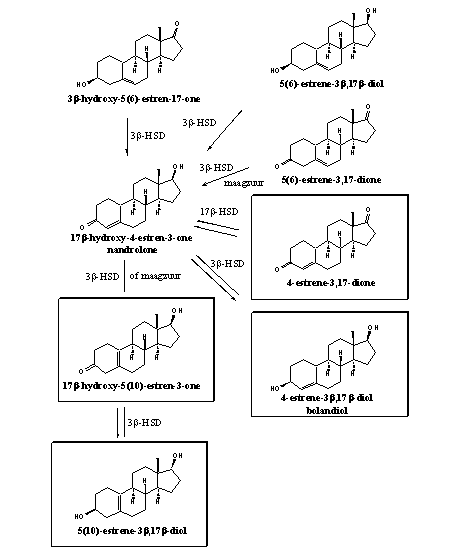
Scheme 3
The biological effects of 4-estrene-3,17-dione (224 mg) en 4-estrene-3,17-diol (120 mg) in resistance trained men have been investigated. They received 344 mg per day of a combination of both prohormones in the indicated ratio during eight weeks. The investigators could not find any difference in strength and body composition between the treated persons and a control group [7].
All possibilities for prohormones shown in scheme 3 are in principle also feasible for nandrolone analogs with extra methyl groups on C7, C11, C2 or C17, or with an ethyl group on C13 or C17 (see Figure 3). Also all combinations of two or more methyl or ethyl groups are possible. The framed steroids in figure 3 have appeared on the market, but we have not yet found prohormones for these compounds. The other steroids in figure 3 are known in the literature but they are not on the market as anabolic steroids or prohormones. The main problem is probably the synthesis of these steroids in larger quantities for ' a reasonable price.
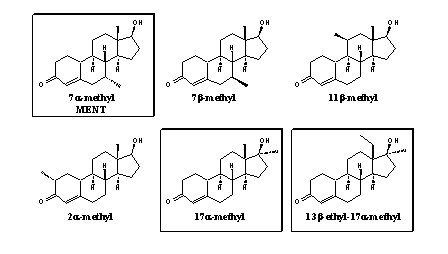
Figure 3
Synthetic chemical procedures, especially for 19-nor steroids, may provide for good and cheep production
of prohormones. The compound with an aromatic ring A and a carbonyl group at C17 in scheme 4 is a
much used starting material for the synthesis of 19-nor steroids. The carbonyl group can react with
organo-metallic reagents to introduce methyl (CH3) or ethynyl groups
(C
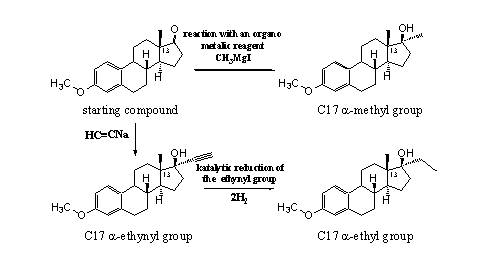
Scheme 4
Reduction of the aromatic ring A of the steroids in Scheme 4, with a so called Birch reduction, will give enol ethers as the first intermediate (see scheme 5). Chemists can isolate this product but they also can convert it to the D5(10)-3one compound by treatment with a weak acid. Also this intermediate can be isolated, but also here it can be converted immediately in the 4-en-3-one compound, the active steroid. Many of such kind of syntheses have been carried out for 19-nor steroids and are mentioned in the elder literature [8] [9] [10] [11] and in old patents [12] [13].
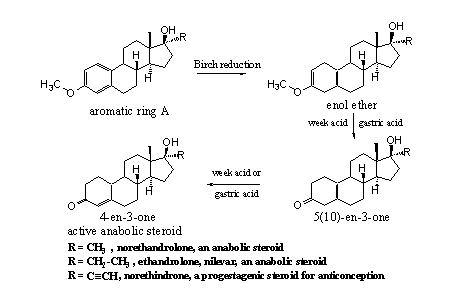
Scheme 5
Gastric acid is a strong acid and is capable to convert the enol ethers and the D5(10)-3one intermediates into the active anabolic steroid, no enzyme is necessary. The intermediate products have only a weak anabolic activity [12], but they may be introduced as prohormones on the market. Users have to take them orally so that gastric acid can do its work. We have not seen these prohormomes yet on the market. They resemble the D5(10) steroid at the bottom of scheme 3.
Also the starting compound with an ethyl group at C13 (see Scheme 6, upper left) is for sale. In a similar way as is shown in schemes 4 and 5, this compound can be converted into anabolic steroids (see Scheme 6). The indicated intermediates are, also in this case, potential prohormones.
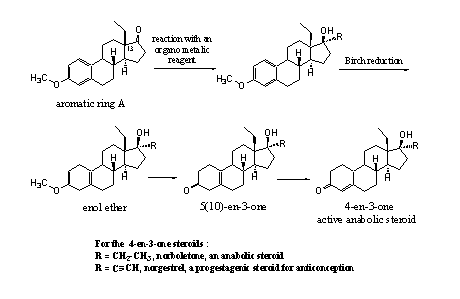
Scheme 6
In the past years several prohormones have been marketed by well known persons like Patrick Arnold, Brock Strasser alias Bruce Kneller, Derek Cornelius, Bill Roberts and Bill Llewellyn. These compounds have become famous in bodybuilder and fitness circles. Some of these prohormones have become a success, some proved to be a disaster for the users.
From the structures of these prohormones (see Scheme 7) it is clear that they fit in the reasoning of this chapter. On the left and right side in Scheme 7 the prohormones are depicted and the anabolic steroids in which they have to be converted by enzymes, are put in the middle. 4-Androstenediol shows anabolic activity themselves although researchers could not confirm this claim for bolandiol [3].
For these well known prohormones it is proven that the desired enzymatic conversion into the real anabolic steroid indeed takes place, but it is still unclear how efficient this conversion really is.
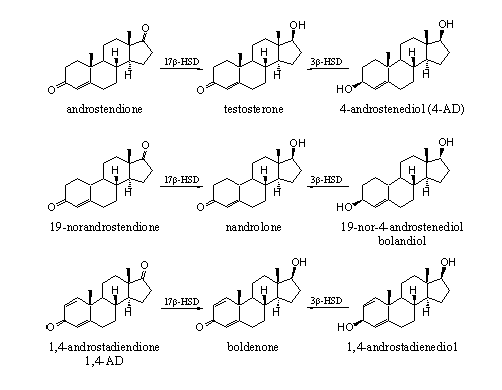
Schema 7
Research on the metabolism of anabolic steroids has been carried out by doping hunters and by the pharmaceutical industry. The latter not only has to show that the drug itself has as few as possible side effects, but they have to show this also for all its metabolites.
The analytical chemical search of doping hunters nowadays is not only aiming at the forbidden anabolic steroid itself, but also on its metabolites [14] [15] [16]. With the help of these metabolites doping abuse can be proven with better accuracy and sometimes also during a longer period after stopping of intake. For this reason the metabolism of a fair number of prohormones, normal anabolic steroids and designer steroids [17] has been investigated.
Meanwhile it will be clear that from a chemical point of view there are many possibilities for new prohormones. It is not a surprise that regularly new prohormones, often advertised as a designer supplement, appear on the market. Producers advertise the new prohormone as legal and they assume that the necessary enzymatic transformations into the real anabolic steroid will take place. They seldom really prove this. The possible activity and the side effects of the prohormone itself mostly never have been investigated. Only scientific research can establish this. Producers simply bring the prohormone on the market.
The user is the guinea pig.
[1] Greene R.S. 08/01/1999.
[2] Uralets V.P., and Gillette P.A. Journal of Analytical Toxicology (2000) 24, 188-193.
[3] Tseng Y.L., Kuo F-H, and Sun K-H. Journal of Analytical Toxicology (2005) 29, 124-134.
[4] Baume M., Mahler N., Kamber M., Mangin P., and Saugy M. Scand. J. Med. Sci. Sports (2006) 16, 41-48.
[5] Ranjith H., Dhamaratne W., Kilgor J.L., Roitman E., Shackleton C. and Caspi E. Journal of the Chemical Society (1993) 1529-1535.
[6] Nes W.R., Loeser E., Kirdani R. and Marsh J. Tetrahedron (1963) 19 299-307.
[7] Van Gammeren D., Falk D. and Antonio J. Nutrition (2002) 18, 734-737.
[8] Colton F.B., Nysted L.N., Riegel B., and Raymond A.L. Journal of the American Chemical Society (1957) 79 1123-1127.
[9] Philips D.K., Wickham P.P., Potts G.O., and Aaron A. Journal of the Chemical Society, Perkin transactions (1968) 11, 924-928.
[10] Baier H., Dürner G., and Quinkert G. Helvetica Chimica Acta (1985) 68 1054-1068.
[11] Djerassi C. Steroids 1992 (57) 631-641.
[12] Colton F.B., United States patent nr 2.905.676.
[13] Clinton R.O., and Christinasen R.G. United States patent nr 3.076.826.
[14] Van Eenoo P., and Delbeke F.T. Journal of Steroid Biochem. and Mol. Biochem. (2006) 101, 161-178.
[15] Schanzer W., and Thevis M. Chemie Unserer Zeit (2004) 38, 230-241.
[16] Thieme D., Anielski P., Grosse J., Sachs H., and Mueller R.K. Analytica Chimica Acta (2003) 483, 299-306.
[17] Levesque J-F., Templeton E., Trimble L., Berthelette C., and Chauret N. Analytical Chemistry (2005) 77, 3164-3172.
|
|