Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 1/6/2007
14. The Working Mechanism of Aromatase
Aede de Groot, Willem Koert
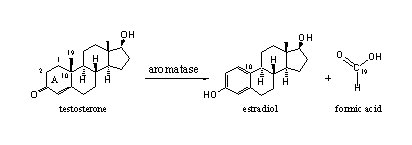
In this chapter we will deal with the working mechanism of the enzyme aromatase. In the chapters 10 and 11 we already have presented the enzyme aromatase, but now we will discuss its working mechanism in depth. This will explain why some steroid hormones aromatize and why others are not converted into estradiol at all.
All anabolic steroids with a similar structure in ring A as testosterone or A-dione can in principle be transformed by aromatase to an estrogen with an aromatic ring A as in estrone or estradiol.
The oxidative removal of the C19-methyl group is catalyzed by a complex of cytochrome P450 enzymes with the code cytochrome P450aromatase or aromatase. The C19 methyl group leaves the molecule as formic acid (see Scheme 1)
Scheme 1
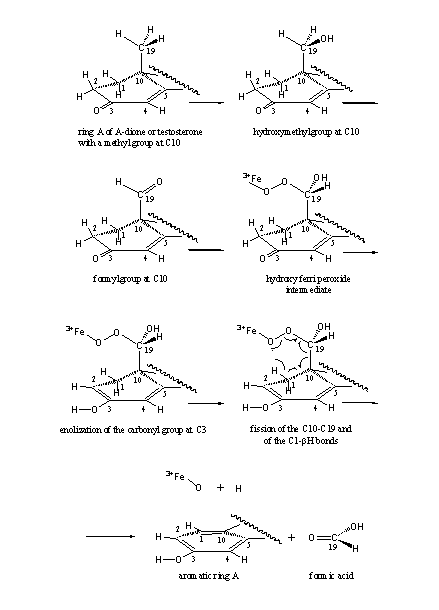
First the methyl group (CH3) is oxidized to a hydroxymethyl group (H2C-OH) and next to a formyl group (HC=O). It is assumed that now a 19-hydroxy-19-ferri peroxide intermediate is formed with the help of a co-enzyme. The fission of the C10-C19 bond takes place in this intermediate, together with the abstraction of the bH-atom from C1.
The abstraction of this bH-atom from C1 is facilitated by enolisation of the C3 carbonyl group - enolisation will be explained hereafter-. The formation of a stable aromatic ring is an extra driving force. In Scheme 2 the whole process is depicted in spatial structures.
Scheme2
Three structural elements have to be present in ring A to ensure an easy aromatisation reaction catalysed by aromatase.
1) The carbonyl group at C3 2) The D4 double bond 3) The C19-methyl group
There has to be a carbonyl group at C3.
Aromatase catalyzes the fission of the bH-atom of C1, as shown in Scheme 2. Enolisation of the carbonyl group facilitates this fission. Before we tell the reason for this, we have to explain the terms enol and enolisation
The name enol is a combination of the suffixes ene for double bonds and ol for hydroxyl compounds and indicates the two elements in this functional group.
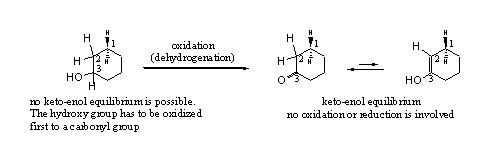
Enolisation is a spontaneous equilibrium in all carbonyl compounds. The equilibrium usually is on the side of the carbonyl compound 2 (the keto compound) and only little of the enol3 (the enol compound) is present. In cyclic carbonyl compounds enolisation is easier and a few percent of the enol may be present (see Scheme 3).
Scheme 3
A keto-enol equilibrium is a simple movement of a H-atom from the C-atom next to the carbonyl group (C2), to the O-atom of the carbonyl group. Simultaneously the double bond shifts from a C=O to a C=C in the ring. Enolisation does not involve oxidation or reduction, it is just a change in place of functional groups. It is good to notice that a hydroxyl group 1, can not enolise because there is no double bond that can shift.
After the shift of the double bond in the ring, the H-atoms at C1 are in a different position. In the keto compound the H-atoms at C1 are isolated from the C=O at C3, because C2 is in between. In the enol compound C1 is located directly next to the C=C double bond in the ring and this makes the H-atoms at C1 more reactive and easier to abstract.
There has to be a D4-double bond
The D4-double bond is one of the three double bonds that are necessary to complete the aromatic system. When there is no D4-double bond, then there will be no aromat.
There has to be a C19 methyl group
The C19 methyl group and the bH-atom leave the molecule together and are replaced by the third double bond at the top of ring A.
Since all three structural elements in ring A are necessary for an easy aromatisation by the enzyme aromatase, it is not really difficult to inhibit this reaction partly or completely. We now will see how that has been carried out.
Steroids without a D4-double bond
The D4- double bond is one of the three double bonds that are necessary to complete the aromatic system. When this double bond left out or put on a different place, then there will be no aromat. There is a fair number of anabolic steroids without a D4-double bond, some are shown in Figures 1 and 2.
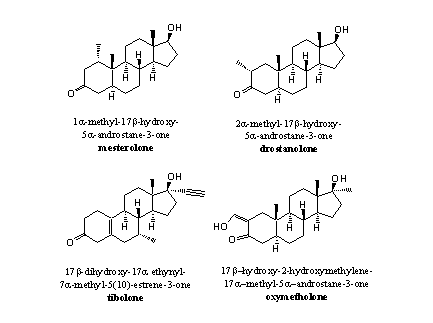
Figure 1
Of course the structure of the anabolic steroid has changed by omitting this D4-double bond. In the case of testosterone you get dihydrotestosterone and this has unwanted androgenic activities. It is not always clear whether the omission of the D4-double bond in other anabolic steroids results in comparable changes from anabolic to androgenic activity.
Omission of the D4-double bond also makes the carbonyl group more vulnerable for reduction to a hydroxyl group. Anabolic steroids with a C3-hydroxyl group are often less active and can be removed easily as a glucuronate or sulphate.
Steroids without a C3 carbonyl group
Simple omission of the carbonyl group at C3 is not a much practised solution. This carbonyl group is an important hydrogen bond acceptor and contributes significantly to bonding of the steroid to the receptor. However, bolenol and ethylestrenol (see Figure 2) are reasonably active anabolic steroids without this C3 carbonyl group. Most anabolic steroids do have a hydroxyl group, a carbonyl group or another functional group that can contribute to binding of the steroid to the receptor through hydrogen bonding (see Figures 1, 2 and 3).
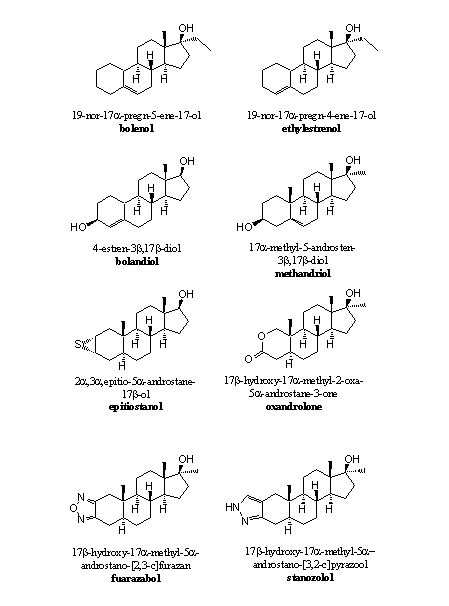
Figure 2
The C3 carbonyl Group is replaced by a hydroxyl group
This is a realistic possibility. A hydroxyl group can not enolise and consequently there will be one double bond short for an aromat. Also the H-atoms at C1 will not be activated for abstraction. For these two reasons, aromatisation will be prevented efficiently. The hydroxyl group at C3 can participate in hydrogen bond formation for better bonding of the anabolic steroid to the receptor, although a bit less efficient then a carbonyl group.
A dehydrogenase may oxidise the hydroxyl group to a carbonyl group and then aromatisation becomes possible again. Examples of anabolic steroids with a hydroxyl group at C3 are bolandriol and methandriol (see Figure 2).
The C3 carbonyl Group can be replaced by a group which can not enolise in the direction of C2.
Anabolic steroids like epithiostanol (with a weak hydrogen bond) or oxandrolon circumvent in such a way the aromatisation problem. These groups can act as a hydrogen bond acceptor and contribute to bonding of the steroid to the receptor. In these anabolics also the D4-double bond is absent and that is also a reason why they do not aromatize.
The attachment of a small heterocyclic aromatic ring to ring A.
The attachment of a heterocyclic ring with a N- or O-atom like in furazabol or stanozolol (see Figure 2) to ring A, has been proven a good solution to prevent aromatisation. These heterocyclic rings are aromatic themselves and reasonably stable. The N-atom at the bottom of the ring may act as a hydrogen bond acceptor. Also in these anabolics the D4-double bond is absent.
Omit the methyl group at C10
The presence of the C19 methyl group, its oxidation to a formyl group and the fission of the C10-C19 and the C1 bH-bonds are all necessary elements in the construction of the C10=C1 double bond in ring A by the enzyme aromatase. The simple omission of this methyl group is therefore a solution to prevent aromatisation. This solution has been applied in anabolic steroids like nandrolone and other 19-nor steroids (see Figure 3)
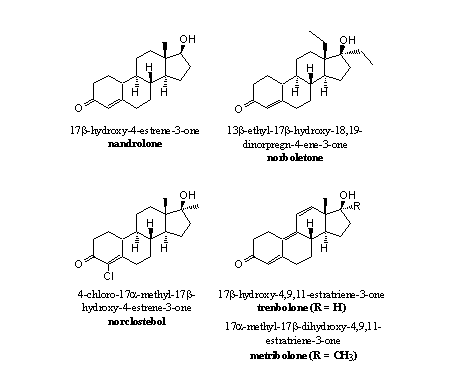
Figure 3
In principle it is possible to aromatise ring A of nandrolone, norboletone or norclostebol but this has to be catalysed by another enzyme and not by aromatase. To aromatise ring A of nandrolone, an H-atom has to be removed from C10 and from C1 and replaced by a double bond. This is a dehydrogenation that may take place. From experiments we know that nandrolone does aromatise to a small extend.
Steroids with a methyl Group at C1
An extra methyl group at C1 will hinder the removal of the bH-atom by aromatase. There simply is less space for the formation of the 19-hydroxy-19-ferri peroxide intermediate. Mesterolone is an example of such a steroid. When there is no H-atom present at C1 like in metenolone, it is clear that it also can not be removed and no aromatisation will take place (see Figure 4).
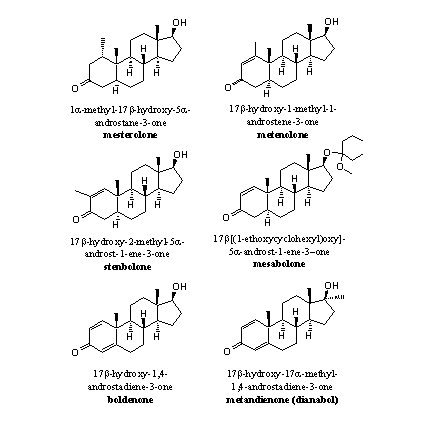
Figure 4
Steroids with a D1-double bond
Examples of that type of steroids are metenolone, stenbolone and mesabolone. In these steroids aromatisation becomes impossible for geometrical reasons. After the fission of the methyl group and the H-atom a system of two cumulative double bonds would result, between C10=C1 and C1=C2 (see Scheme 4).
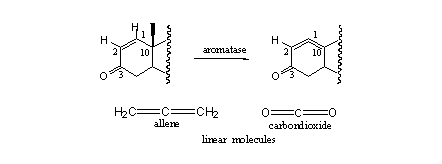
Scheme 4
Such a system is in principle possible but the geometry of it would be linear. The system is called an allene and it is similar to the bonding in carbondioxide, which is also a linear molecule. Linear structural elements can not exist in a six membered ring, because of the bent geometry with an internal angle of 120o that would be necessary in the latter. This would create too much strain in the molecule.
A D1-double bond can prevent aromatisation and at the same time stabilize the carbonyl group for reduction through formation of a conjugated system. Also here the shape of the molecule is changed, which will have consequences for its bonding with the androgen receptor.
From experiments we know that anabolic steroids with two double bonds in ring A like boldenone and dianabol do arimatise to a certain extend. A reasonable explanation for this phenomenon can be given (see below), but also in this process the enzyme aromatase probably plays a limited role.
We have shown that many structural adaptations have been applied to prevent aromatization. In many of the examples even more then one of these adaptations are present. In the sixties scientists have investigated the aromatization of anabolic steroids in human placenta tissue [1][2], and the results give a good impression about the scope and limitations of the enzyme aromatase.
In figure 5 the structures of the steroids and the results of these investigations are summarized. The bold number under the structure of the steroid indicates the relative reactivity of the steroid in the aromatization reaction. The reactivity of 4-androsten-3,17-dione (A-dione) (1), the natural substrate of aromatase, is fixed at 100.
- Steroids 1 and 2, A-dione and testosteronee, are the natural substrates of aromatase. The reactivity of these natural substrates has been fixed at 100.
- Steroid 3 is the first intermediate in the aromatization reaction (see Scheme 2). The first oxidation has taken place already in this compound and this is often the most difficult step. It helps when this has been carried out already. It is therefore not surprising that this intermediate can be aromatized quickly.
- The reactivity of steroid 4 (DHEA) is also not unexpected. DHEA is the natural precursor of estrone in the biosynthesis and it is probably metabolized in a normal way in this test system to A-dione and next to estrone.
- The influence of extra hydroxyl groups on aromatization is not so easy to explain. An 11a-hydroxyl group apparently is no problem (see steroid 5). The 2b- and 6b-hydroxyl groups (steroids 6 and 7) aromatize only in 15 and 20 % and when the hydroxyl groups are in the 2a, 1a or 11b (steroids 8, 9 and 10), no aromatization takes place at all. We do not have an explanation for this behavior. In principle all steroids can aromatize, because all structural preconditions are present in ring A.
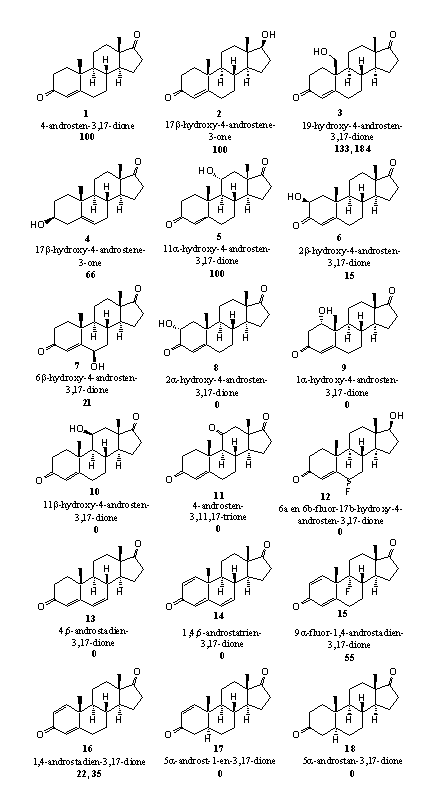
Figuur 5
- We also do not have an explanation for the fact that steroids 11 and 12 do not aromatize. Probably the enzyme aromatase does not accept these compounds as a substrate but it is difficult to indicate why not.
- An extra double bond at C6 apparently also is an objection for aromatization as is shown by the behavior of steroids 13 and 14. Also in these cases the enzyme probably does not accept them as a substrate.
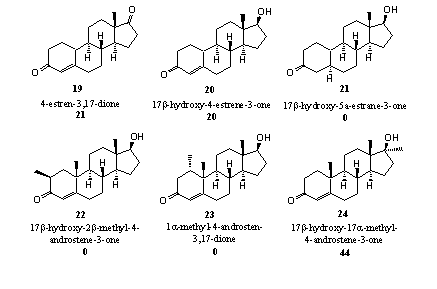
- A certain amount of aromatization does occur in the D1,4 steroids 15 and 16. Probably the aromatization reaction follows a different pathway here, in which aromatase only plays a minor role. In organic chemistry a reaction is known which can take place in the hydroxyl and carbonyl intermediates of steroids 15 and 16. These reactions are shown in Scheme 5 and it is clear that the 19-hydroxy-19-ferri peroxide intermediate is not necessary to achieve aromatization. In the left column we see that the hydroxyl compound can simply loose formaldehyde with help of an hydroxide ion (OH-), followed by aromatization of ring A. In the right column a similar type of reaction can take place with fission of formic acid after reaction of the carbonyl group with an hydroxide ion (OH-). The presented mechanism is speculative but realistic.
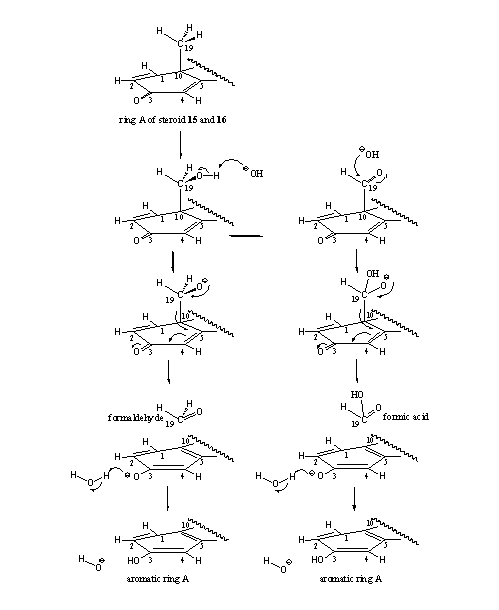
Scheme 5
- It is no surprise that steroids 17 and 18 do not aromatize because the double bond at C4 is missing.
- It is however a bit of a surprise that the nandrolone compounds 19 and 20 do aromatize for a small part. In principle this is of course possible because the H-atoms at C10 and C1 can be replaced by a double bond. After enolization of the C3 carbonyl group an aromat is obtained. It is reasonable to assume that aromatase is not involved in these reactions.
- It is no surprise again that steroid 21 does not aromatize because also here the C4 double bond is missing.
- Extra methyl groups in ring A as in steroids 22 and 23 apparently cause enough steric hindrance to prevent aromatization. These steroids do not fit well enough in the reaction pocket of the enzyme and the catalytic groups can not act properly. When the methyl group is placed further away from the reaction center as in steroid 24, aromatization becomes possible again. Ring A in methyltestosterone 24 is chemically identical to ring A in testosterone itself and aromatization can take place as indicated in scheme 2.
It is clear from these examples and experimental results that it is not really straightforward to predict the behavior of a steroid, just based on its chemical structure. Always biological experiments in living systems will be necessary to verify the predictions.
Prevention of aromatisation is important for the treatment of estrogen dependent cancers and for that reason much research has been carried out to find aromatase blockers. Also for bodybuilders this is of interest because these blockers may prevent gynaecomastia in man resulting from anabolic steroid abuse.
[1] Ryan K.J., J. Biol. Chem. (1959) 234, 268-272.
[2] Gual C., Morato T., Hayano M., Gut M. and Dorfman R.I., Endocrinology (1962) 71,
920-925.
|
|